Question: PLEASE ANSWER ALL 1) For TLC, you need to use a transfer solvent (solvent to dissolve your compound for spotting on your TLC plates; e.g.
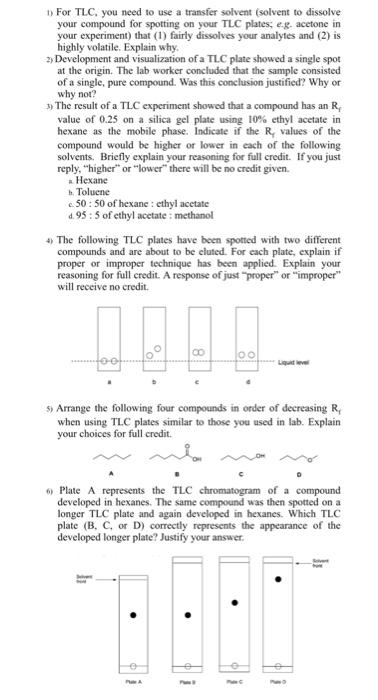

1) For TLC, you need to use a transfer solvent (solvent to dissolve your compound for spotting on your TLC plates; e.g. acetone in your experiment) that (1) fairly dissolves your analyftes and (2) is highly volatile. Explain why. 2) Development and visualization of a TLC plate showed a single spot at the origin. The lab worker concluded that the sample consisted of a single, pure compound. Was this conclusion justified? Why or why not? 5) The result of a TLC experiment showed that a compound has an Rf value of 0.25 on a silica gel plate using 10% ethyl acetate in hexane as the mobile phase. Indicate if the R, values of the compound would be higher or lower in each of the following solvents. Briefly explain your reasoning for full credit. If you just reply, "higher" or "lower" there will be no credit given. - Hexane b. Toluene c 50 : 50 of hexane : ethyl acetate d. 95:5 of ethyl acctate : methanol 4) The following TLC plates have been spotted with two different compounds and are about to be cluted. For each plate, explain if proper or improper technique has been applied. Explain your reasoning for full credit. A response of just "proper" or "improper" will reccive no credit. 5) Arrange the following four compounds in order of decreasing Rr when using TLC plates similar to those you used in lab. Explain your choices for full credit. 6) Plate A represents the TLC chromatogram of a compound developed in hexanes. The same compound was then spotted on a longer TLC plate and again developed in hexanes. Which TLC plate (B,C, or D ) correctly represents the appearance of the developed longer plate? Justify your answer. 7) You are trying to find a TLC solvent system that will separate the compounds X, Y, and Z. You developed the compounds on a TLC plate using 95:5 hexanes: ethyl acetate as the eluting solvent and obtained the following chromatogram: a. Is this solvent system appropriate for separating X, Y, and Z? Why or why not? b. How could you change the solvent system to give you a better separation of X, Y, and Z? 8) You are given a sample that is believed to be either cis- or transstilbene. Would TLC be a good method of determining which compound that you have? Why or why not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


