Question: please answer all and show work. 8. How many grams of the first reactant in each of the following chemical equations would be needed to
please answer all and show work.
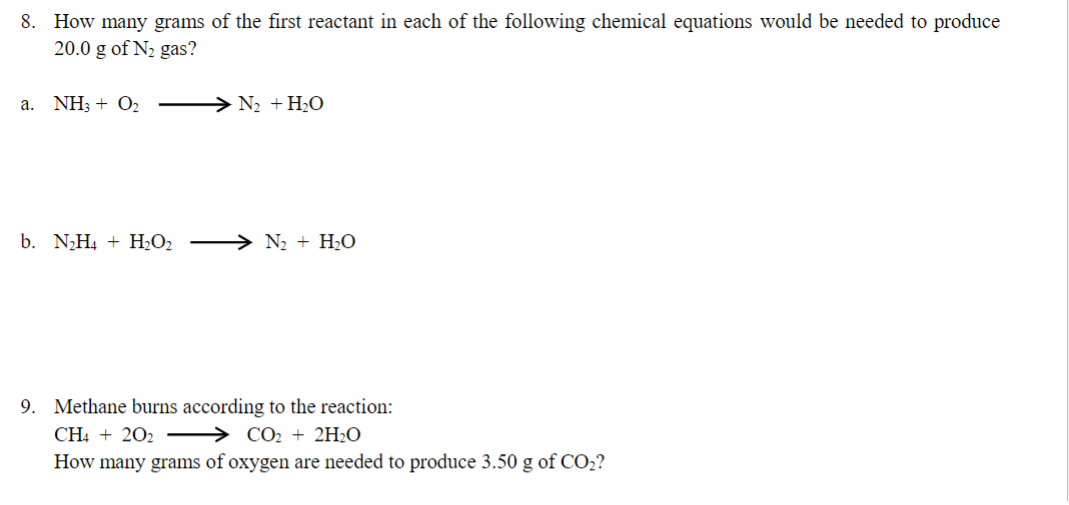
8. How many grams of the first reactant in each of the following chemical equations would be needed to produce 20.0g of N2 gas? a. NH3+O2N2+H2O b. N2H4+H2O2N2+H2O 9. Methane burns according to the reaction: CH4+2O2CO2+2H2O How many grams of oxygen are needed to produce 3.50g of CO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


