Question: please answer all thank you so much, i will leave a like 3. Make an energy diagram for the heat formation of one mole of

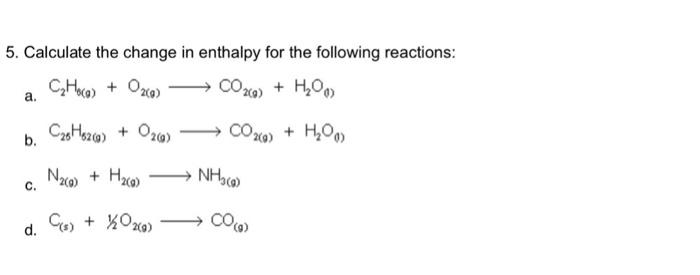
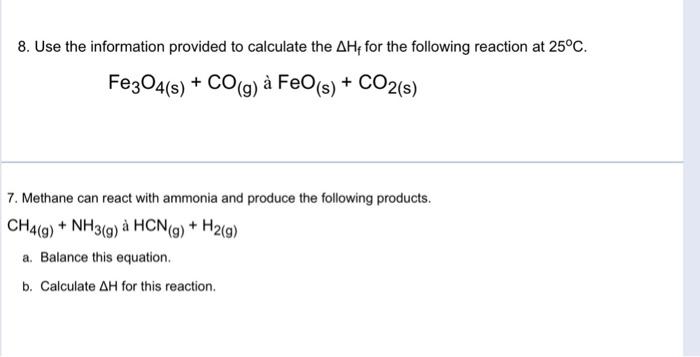
3. Make an energy diagram for the heat formation of one mole of each of the following substances: c. N2+4H2 2NH4+ 5. Calculate the change in enthalpy for the following reactions: a. C2H0(0)+O2(0)CO2(0)+H2O0) b. C25H62()+O2(g)CO2()+H2O0) c. N2()+H2()NH3() d. C(s)+1/2O2()CO(g) 8. Use the information provided to calculate the Hf for the following reaction at 25C. Fe3O4(s)+CO(g)aFeO(s)+CO2(s) 7. Methane can react with ammonia and produce the following products. CH4(g)+NH3(g)aHCN(g)+H2(g) a. Balance this equation. b. Calculate H for this reaction. 3. Make an energy diagram for the heat formation of one mole of each of the following substances: c. N2+4H2 2NH4+ 5. Calculate the change in enthalpy for the following reactions: a. C2H0(0)+O2(0)CO2(0)+H2O0) b. C25H62()+O2(g)CO2()+H2O0) c. N2()+H2()NH3() d. C(s)+1/2O2()CO(g) 8. Use the information provided to calculate the Hf for the following reaction at 25C. Fe3O4(s)+CO(g)aFeO(s)+CO2(s) 7. Methane can react with ammonia and produce the following products. CH4(g)+NH3(g)aHCN(g)+H2(g) a. Balance this equation. b. Calculate H for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


