Question: please answer all thank you very much Consider the hypothetical reaction shown below: A(g) + 5 B(8) 5 C(g) + D (1) The equilibrium constant,
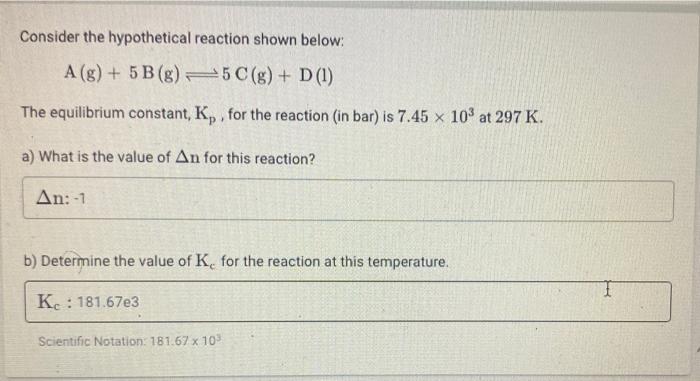
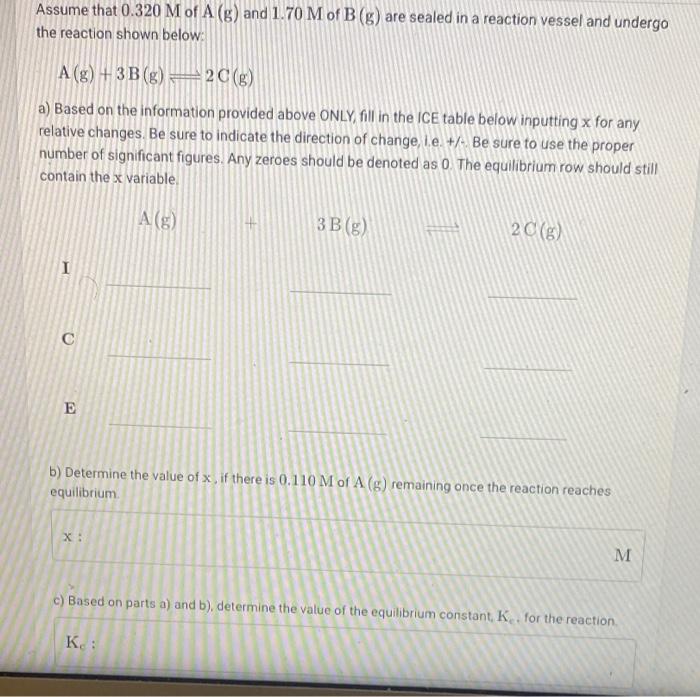
Consider the hypothetical reaction shown below: A(g) + 5 B(8) 5 C(g) + D (1) The equilibrium constant, Ky, for the reaction (in bar) is 7.45 x 103 at 297 K. X a) What is the value of An for this reaction? An:-1 b) Determine the value of K for the reaction at this temperature. K.: 181.67e3 Scientific Notation: 181.67 x 103 Assume that 0.320 M of A (g) and 1.70 M of B(g) are sealed in a reaction vessel and undergo the reaction shown below: A (g) + 3B (g) = 2(g) a) Based on the information provided above ONLY fill in the ICE table below inputting x for any relative changes. Be sure to indicate the direction of change, i.e. +. Be sure to use the proper number of significant figures. Any zeroes should be denoted as 0 The equilibrium row should still contain the x variable A (3) (9 3 B (8) 20(8) I I E b) Determine the value of x, if there is 0.110 M of A (8) remaining once the reaction reaches equilibrium X: M M c) Based on parts a) and b), determine the value of the equilibrium constant, K. for the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


