Question: please answer both and write nice please. also write the answer the format the questions askes. thank you How many liters of 0.305 MKPO, solution
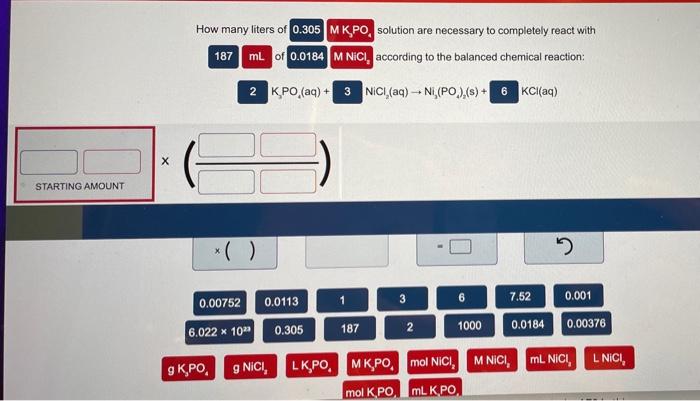
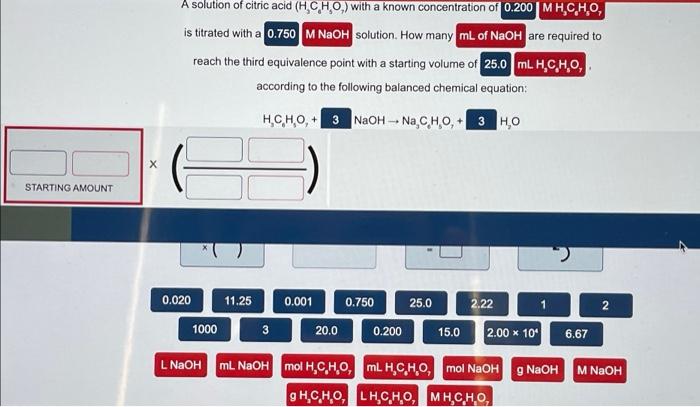
How many liters of 0.305 MKPO, solution are necessary to completely react with 187 mL of 0.0184 MNICI, according to the balanced chemical reaction: 2 K PO (aq) + 3 NICI (aq) -- NI,(PO),(s) + 6 KCl(aq) STARTING AMOUNT ( ) 0.00752 1 3 7.52 0.001 0.0113 0.305 187 2 6.022 * 102 1000 0.0184 0.00376 M NICI, mL. NICI, L NICI g. g NICI, LK,PO MK PO mol NICI, mol K PO mL K PO A solution of citric acid (H,CHO,) with a known concentration of 0.200 MH.C.H.0, is titrated with a 0.750 M NaOH solution. How many mL of NaOH are required to reach the third equivalence point with a starting volume of 25.0 ML. H.C,H,O, according to the following balanced chemical equation: HC,H,O + 3 NaOH - Na C HO + | STARTING AMOUNT 0.020 11.25 25.0 2.22 2 0.001 20.0 0.750 0.200 1000 3 15.0 2.00 * 109 6.67 M NaOH LNaOH ml NaOH mol H,C,H,O, MLH.C.H.O, mol NaOH g NaOH g H,CH, H,CHO, MH,CHO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


