Question: For the reaction A(g) + B(g) + C(g), Kp at 400 Cis 1.5 x 10 and Kp at 600C is 6 x 10-3. Then
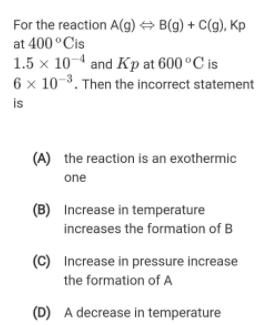
For the reaction A(g) + B(g) + C(g), Kp at 400 Cis 1.5 x 10 and Kp at 600C is 6 x 10-3. Then the incorrect statement is (A) the reaction is an exothermic one (B) Increase in temperature increases the formation of B (C) Increase in pressure increase the formation of A (D) A decrease in temperature
Step by Step Solution
3.55 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


