Question: Please answer question 1 & 2 A reaction has a rate constant of 2.33105s1 at 341K and a rate constant of 0.344s1 at 468K. What

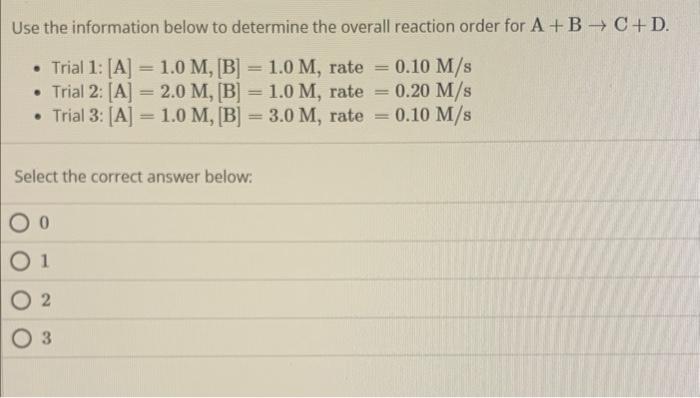
A reaction has a rate constant of 2.33105s1 at 341K and a rate constant of 0.344s1 at 468K. What is the activation energy for the reaction based on the Arrhenius equation? Ea=R[(T21)(T11)lnk2lnk1] - Use R=8.314molKJ Select the correct answer below: 2.31104molJ1.00105molJ7.25103molJ4.21104molJ Use the information below to determine the overall reaction order for A+BC+D. - Trial 1: [A]=1.0M,[B]=1.0M, rate =0.10M/s - Trial 2:[A]=2.0M,[B]=1.0M, rate =0.20M/s - Trial 3:[A]=1.0M,[B]=3.0M, rate =0.10M/s Select the correct answer below: 0 1 2 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


