Question: Please answer sections C & D! A. Determination of Reaction Times Dole 8 Lab Sec 180S Molar concentration of Na,s,o, 0.02 Volume of Na 8,0,
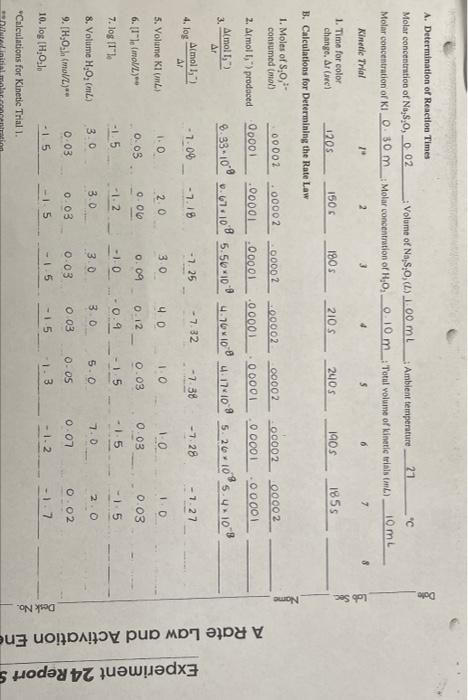

A. Determination of Reaction Times Dole 8 Lab Sec 180S Molar concentration of Na,s,o, 0.02 Volume of Na 8,0, (L) 1.00 mL Ambient temperature 21 "c Molar concentration of Kl 030 m Molar concentration of H.0.0.10 m Total volume of kinetic trials 10mL Kinetle Tal 2 3 4 $ 6 7 1. Time for color change, are 1205 1506 210 S 2405 1905 1855 B. Calculations for Determining the Rate Law 1. Moles of so, consumed into 00002 00002 00002 00002 00002 00002 00002 2. Almol 1,"produced 00001 00001 .00001 00001 00001 0 0001 00001 Amoll) 3. 8.33-10 0.67:108 5.56-100 4.76 100 4.17.10 5.20*105.410-9 ar Name Amol !) 4. log ar -7.08 -7.18 -7.25 -7.32 -7.38 -7.28 -7.27 10 5. Volume KI 30 20 40 10 10 10 0.06 0.03 6. [1" (mol/L) 0.09 012 0.03 0.03 0.03 A Rate Law and Activation Ene Experiment 24 Reports 7. log (1 -1.5 -1.2 -10 -0.9 -1.5 - 15 -15 3.0 8. Volume H,O, 3.0 3.0 3o 5.0 2.0 7.0 0.03 9. [H,Oah (mol/L. 0.03 O 03 0.03 0OS 007 0.02 - 15 - 5 - 15 10. log (H,0:1 -1.5 1.3 - 1.2 Desk No. "Calculations for Kinetic Triall C. Determination of the Reaction Order,p and q, for Each Reactant Instructor's approval of graphs: 1. log (Amol 1, "/A!) versus log 1 lo 2. log (Amol 1,7A/) versus log (H2O2). 3. value of p from graph value of x from graph Write the rate law for the reaction. 7 8 D. Determination of k', the Specific Rate Constant for the Reaction Kinetic Trial 7 2 3 1. Value of ' 2. Average value of ' 3. Standard deviation of 4. Relative standard deviation of k' (%RSD) Appendix B Appendix B A. Determination of Reaction Times Dole 8 Lab Sec 180S Molar concentration of Na,s,o, 0.02 Volume of Na 8,0, (L) 1.00 mL Ambient temperature 21 "c Molar concentration of Kl 030 m Molar concentration of H.0.0.10 m Total volume of kinetic trials 10mL Kinetle Tal 2 3 4 $ 6 7 1. Time for color change, are 1205 1506 210 S 2405 1905 1855 B. Calculations for Determining the Rate Law 1. Moles of so, consumed into 00002 00002 00002 00002 00002 00002 00002 2. Almol 1,"produced 00001 00001 .00001 00001 00001 0 0001 00001 Amoll) 3. 8.33-10 0.67:108 5.56-100 4.76 100 4.17.10 5.20*105.410-9 ar Name Amol !) 4. log ar -7.08 -7.18 -7.25 -7.32 -7.38 -7.28 -7.27 10 5. Volume KI 30 20 40 10 10 10 0.06 0.03 6. [1" (mol/L) 0.09 012 0.03 0.03 0.03 A Rate Law and Activation Ene Experiment 24 Reports 7. log (1 -1.5 -1.2 -10 -0.9 -1.5 - 15 -15 3.0 8. Volume H,O, 3.0 3.0 3o 5.0 2.0 7.0 0.03 9. [H,Oah (mol/L. 0.03 O 03 0.03 0OS 007 0.02 - 15 - 5 - 15 10. log (H,0:1 -1.5 1.3 - 1.2 Desk No. "Calculations for Kinetic Triall C. Determination of the Reaction Order,p and q, for Each Reactant Instructor's approval of graphs: 1. log (Amol 1, "/A!) versus log 1 lo 2. log (Amol 1,7A/) versus log (H2O2). 3. value of p from graph value of x from graph Write the rate law for the reaction. 7 8 D. Determination of k', the Specific Rate Constant for the Reaction Kinetic Trial 7 2 3 1. Value of ' 2. Average value of ' 3. Standard deviation of 4. Relative standard deviation of k' (%RSD) Appendix B Appendix B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


