Question: (C) IF4+ shape octahedral 00 seesaw square pyramidal tetrahedral trigonal pyramidal X hybridization 60 90 109.5 120 X ideal bond angle(s) deviation from ideal
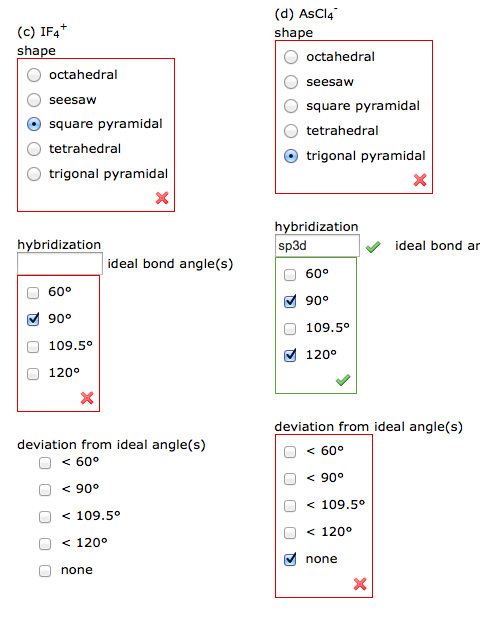
(C) IF4+ shape octahedral 00 seesaw square pyramidal tetrahedral trigonal pyramidal X hybridization 60 90 109.5 120 X ideal bond angle(s) deviation from ideal angle(s) < 60 < 90 < 109.5 < 120 none (d) AsCl4 shape octahedral seesaw square pyramidal tetrahedral trigonal pyramidal hybridization sp3d 60 90 109.5 120 deviation from ideal angle(s) < 60 < 90 < 109.5 < 120 none ideal bond ar X
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
9 Cong f It han sp d hybridisation and its gewoochey shape Should be Trigonal bippramidal Trig... View full answer

Get step-by-step solutions from verified subject matter experts


