Question: Please answer the last 3 questions. Liquid extraction is an operation used to separate the components of a liquid mixture of two or more species.

Please answer the last 3 questions.
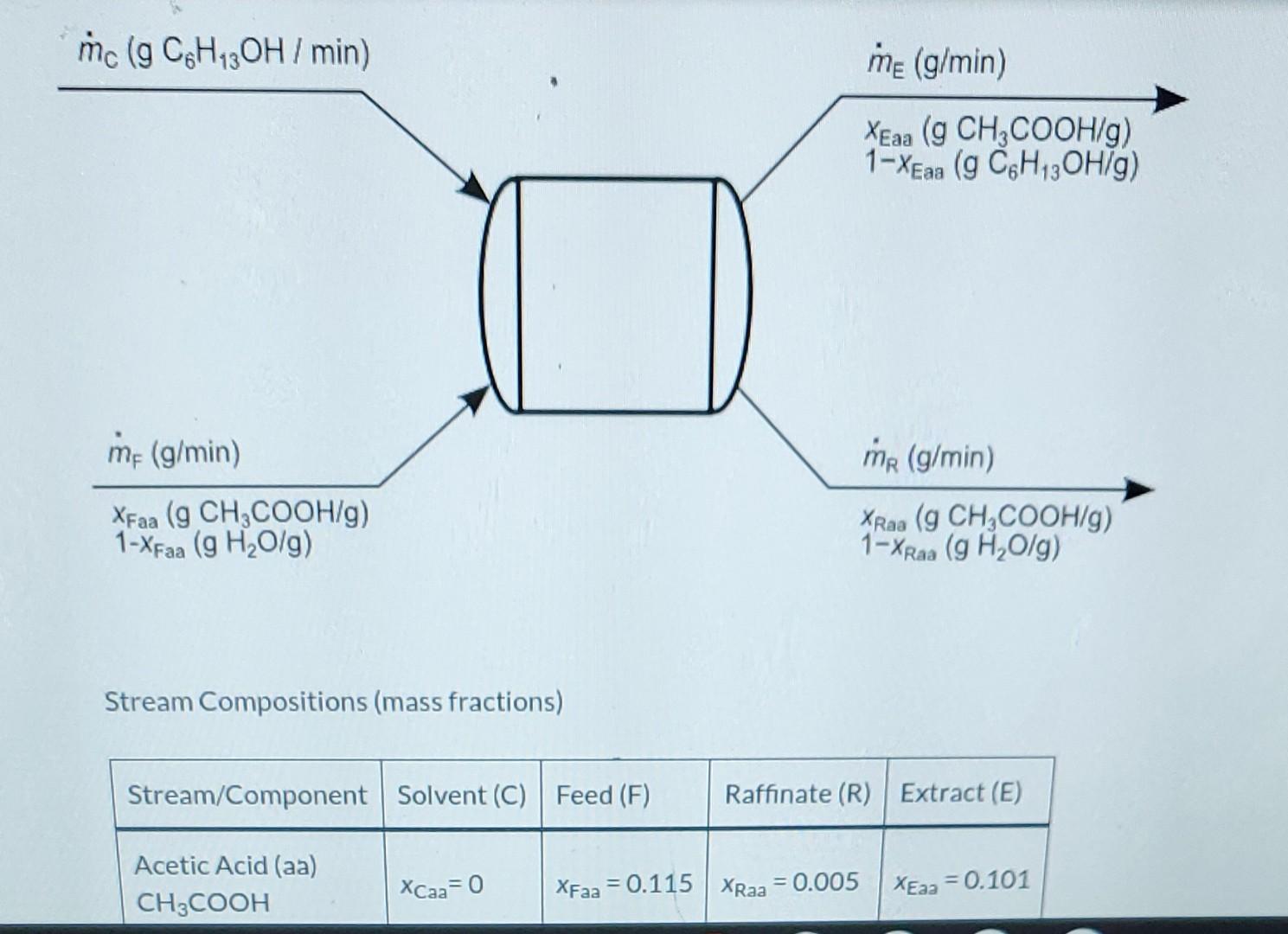
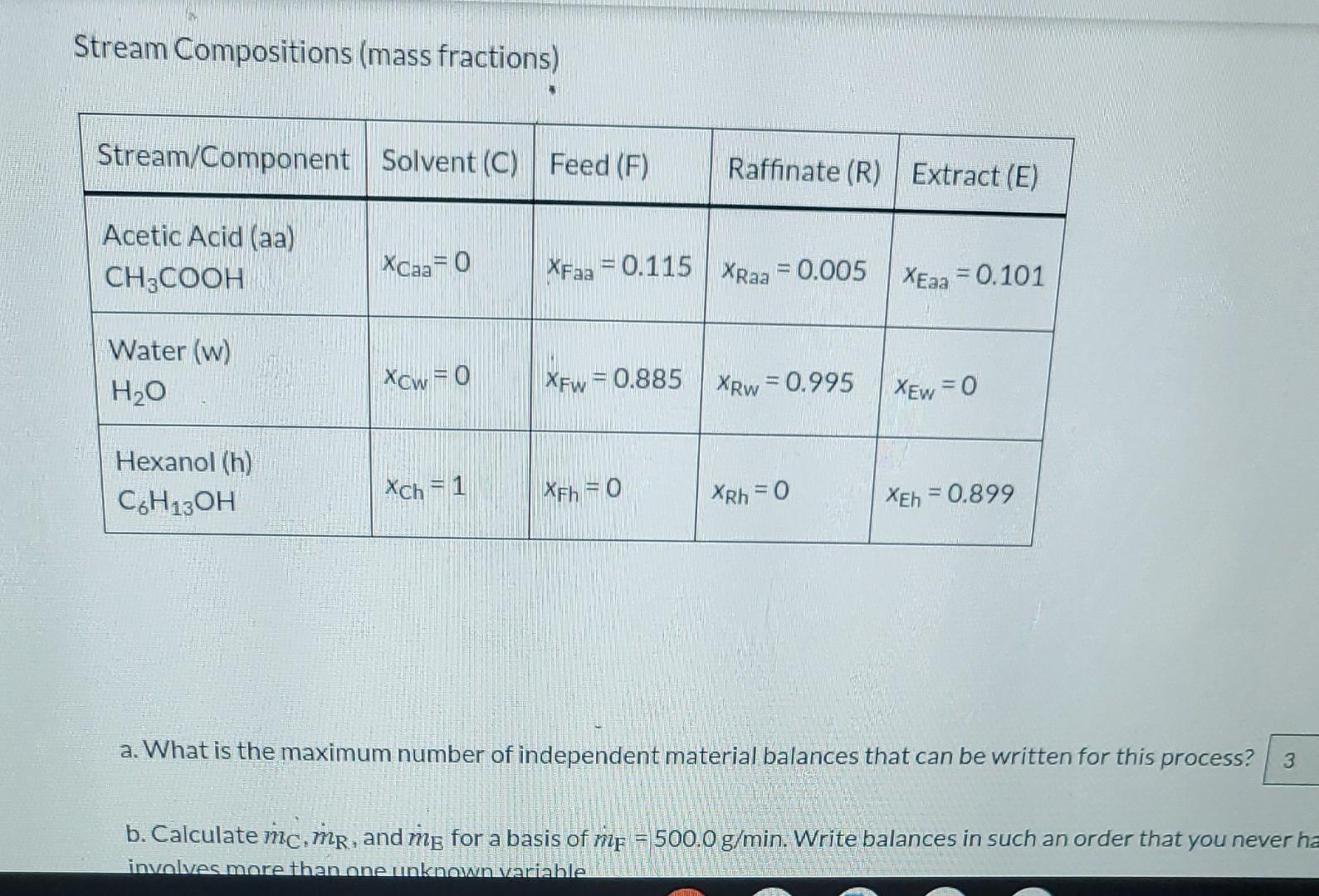
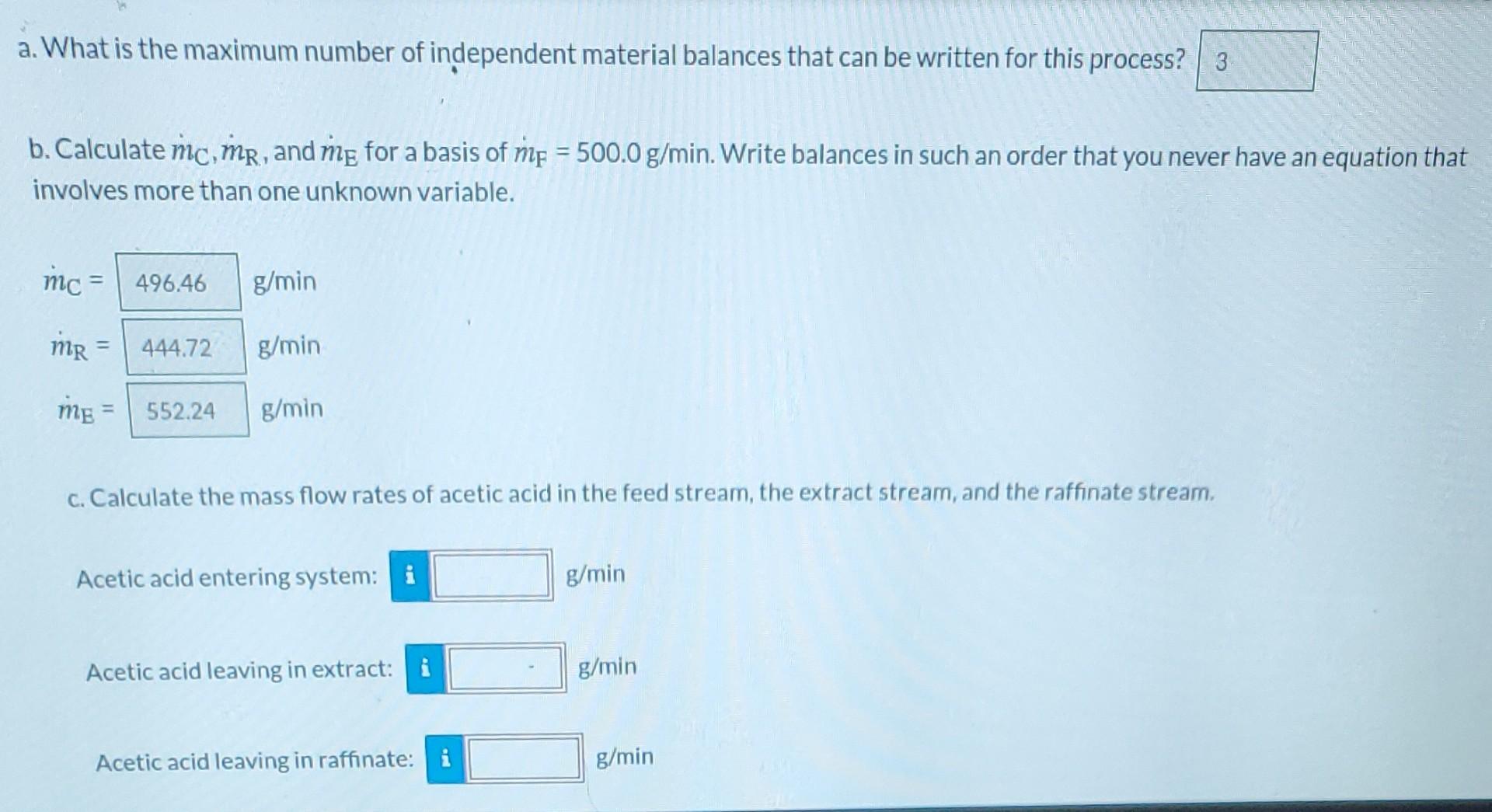
Liquid extraction is an operation used to separate the components of a liquid mixture of two or more species. In the simplest case, the mixture contains two components: a solute (A) and a liquid solvent (B). The mixture is contacted in an agitated vessle with a second liquid solvent (C) that has two key properties: A dissolves in it, and Bis immiscible or nearly immiscible with it. (For example, B may be water, C a hydrocarbon oil, and A a species that has significant solubility in both water and oil.) Some of the A transfers from B to C, and then the B-rich phase (the raffinate) and the C-rich phase (the extract) separate from each other in a settling tank. If the raffinate is then contacted with fresh C in another stage, more A will be transferred from it. This process can be repeated until essentially all of the A has been extracted from the B. Shown below is a flowchart of a process in which acetic acid (aa) is extracted from a mixture of acetic acid and water (w) into 1-hexanol a (h), a liquid immiscible with water. mc (g C6H3OH I min) me (g/min) XEaa (g CH3COOH/g) mc (g C6H, OH / min) me (g/min) XEaa (g CH2COOH/g) 1-XEN (g C6H12OH/g) aa me (g/min) mr (g/min) XFaa (g CH2COOH/g) 1-XFxa (g H2O/g) XRao (g CH,COOH/g) 1-XRaa (g H2O/g) Stream Compositions (mass fractions) Stream/Component Solvent (C) Feed (F) Raffinate (R) Extract (E) Acetic Acid (aa) CH3COOH XCaa= 0 XFaa = 0.115 XRaa = 0.005 XEaa = 0.101 Stream Compositions (mass fractions) Stream/Component Solvent (C) Feed (F) Raffinate (R) | Extract (E) Acetic Acid (aa) CH3COOH Xcaa= 0 XFaa = 0.115 XRaa = 0.005 XEaa = 0.101 Water (w) H2O Xow = 0 XFw = 0.885 XRw = 0.995 XEw = 0 Hexanol (h) C6H130H Xch = 1 XFh = 0 XRh = 0 XEh = 0.899 a. What is the maximum number of independent material balances that can be written for this process? 3 b. Calculate mc.mp, and mg for a basis of me = 500.0 g/min. Write balances in such an order that you never ha involves more than one unknown variable a. What is the maximum number of independent material balances that can be written for this process? 3 b. Calculate mc, mp, and me for a basis of mp = 500.0 g/min. Write balances in such an order that you never have an equation that involves more than one unknown variable. mc 496.46 g/min MR - 444.72 g/min me = 552.24 g/min C. Calculate the mass flow rates of acetic acid in the feed stream, the extract stream, and the raffinate stream, Acetic acid entering system: i g/min Acetic acid leaving in extract: i g/min Acetic acid leaving in raffinate: g/min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


