Question: please answer what you can! volume at equivalence point Volume at one-half equivalence point pKa Ka Average Ka Standard Deviation DATA ANALYSIS ml NaOH PH
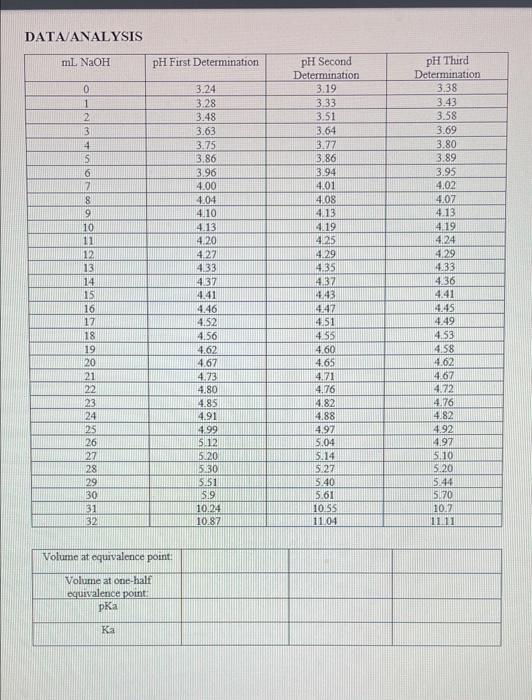
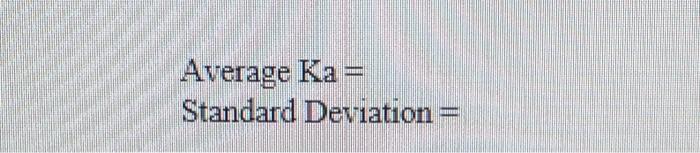
DATA ANALYSIS ml NaOH PH First Determination 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 3.24 3.28 3.48 3.63 3.75 3.86 3.96 4.00 4.04 4.10 4.13 4.20 427 4.33 4.37 4.41 4.46 4.52 4.56 4.62 4.67 4.23 4.80 4.85 4.91 4.99 512 5120 5:30 5.51 59 10.24 10.87 pH Second Determination 3.19 3.33 3.51 3.64 3.77 3.86 3.94 4.01 4.08 4.13 4.19 4.25 4.29 4.35 4.37 443 4.47 4.51 4.55 4.60 4.65 4.71 4.76 4.82 4.88 4.97 5.04 pH Third Determination 3.38 3.43 3.58 3.69 3.80 3.89 3.95 4,02 4.07 4.13 4.19 4.24 4.29 4.33 4.36 4.41 4.45 4.49 4.53 4.58 4.62 4.67 4.72 4.76 4.82 4.92 4.97 5110 5.20 5.44 5.70 10.2 11.11 5.14 5.27 5.40 5.61 10155 11.04 Volume at equivalence point: Volume at one-half equivalence point Ka Average Ka= Standard Deviation =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


