Question: please answer, will rate Put the following acids in order from strongest weak acid to weakest weak acid? The acid and it's Ka value are
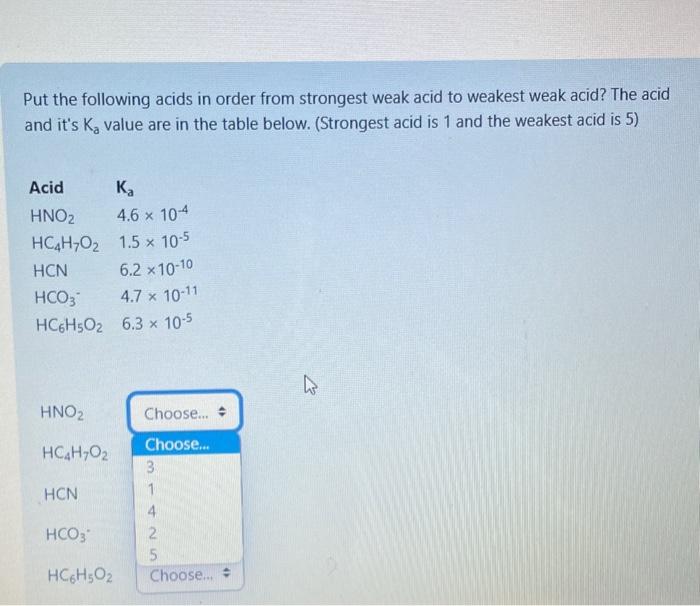
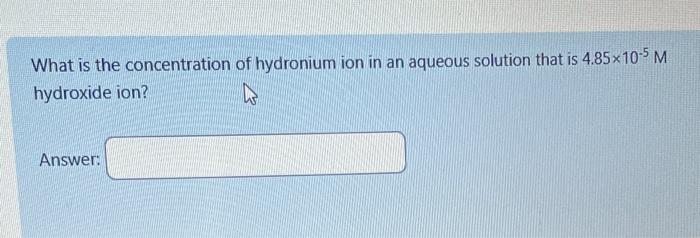
Put the following acids in order from strongest weak acid to weakest weak acid? The acid and it's Ka value are in the table below. (Strongest acid is 1 and the weakest acid is 5) HNO2 What is the concentration of hydronium ion in an aqueous solution that is 4.85105M hydroxide ion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


