Question: Please assist with mass balance for each stream indicated below: Stream 1 : Pure propylene Stream 2 : Air (Oxygen) Stream 3 : Steam(H20) low

Please assist with mass balance for each stream indicated below:
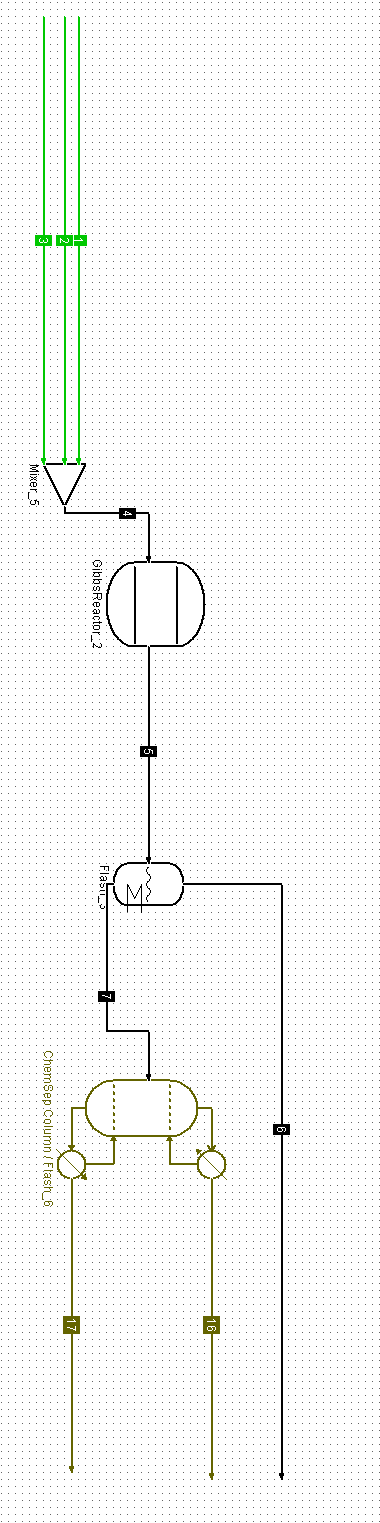
Stream 1 : Pure propylene
Stream 2 : Air (Oxygen)
Stream 3 : Steam(H20) low pressure
Stream 4 : Gibbs reactor feed containing
- 0.05 mole% Propylene
- 0.55 mole % Air
- 0.40 mole % Steam
Gibbs reactor: Temperature vary between 200 - 650 degrees celcius. pressure constant at 4.3 atm
Stream 5 : Reactor effluent / seperation tower feed
- Make use of extent of reaction method to calculate stream composition and molar flow rate
- Contains Acrylic acid, unreacted propylene and by products
Stream 6 : Seperation tower Top
- Stream contains by products and unreacted propylene
Stream 7 : Seperation tower bottoms / Distillation tower feed
- Stream contains acrylic acid and water
Stream 16 :Distillation tower top
- Stream contains water/ waste water stream
Stream 17 : Distillation tower bottom
- Stream contains 99.9mole % acrylic acid (This stream need to be able to produce the 50 000 metric ton of acrylic acid for the year)
PLEASE STATE ALL CALCULATIONS AND MAKE ASSUMPTIONS WHERE INFO IS NOT PROVIDED ON PURPOSE.
Important to show also all stoichiometric coefficients for the two reactions, as this will be required for running a COCO/ASPEN simulation.
Urgently need some help with this, Please!!!!
Design a chemical plant to produce 50000 metric tons per annum of acrylic acid from propylene (assumed pure propylene), air (a source of oxygen), and steam. The compressed air contains water vapor at an amount that would saturate the air at 25C and 1atm. The components are mixed and fed to the reactor at 4.3atm. The mole fractions of the feed are: 0.05 propylene, 0.55 air and 0.4 steam. The reactions occurring withing the reactor are as follows: C3H6+1.5O2C3H4O2+H2O Propylene acrylic acid C3H6+4.5O23CO2+3H2O Propylene Equation (1) is the desired acrylic acid productipn reaction and equation (2) is the undesired propylene combustion reaction. Selectivity and conversion at various temperatures. The plant should operate for 330 days in a year, to allow for shutdown and maintenance. You are required to analyse a simplified acrylic production process and to suggest profitable operating conditions. Evaluate the feasibility of a process to produce 50000 tonne/year of ammonia by producing the following: 1. A conceptual design of the process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


