Question: please solve this problem on polymath and explain how did you do it, don't coppy other tutors please. P11-4, OEQ (Old Exam Question). The elementary,
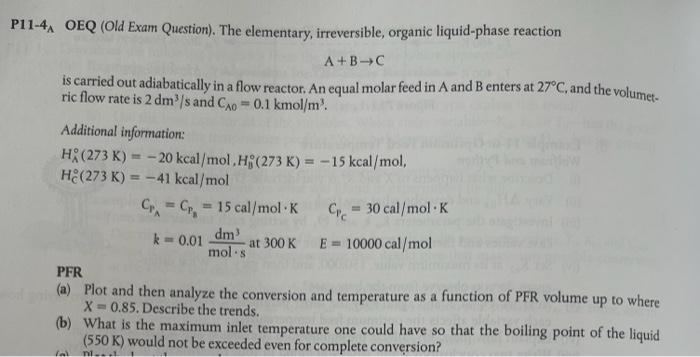
P11-4, OEQ (Old Exam Question). The elementary, irreversible, organic liquid-phase reaction A+B-C is carried out adiabatically in a flow reactor. An equal molar feed in A and B enters at 27C, and the volumet- ric flow rate is 2 dms and Co = 0.1 kmol/ml. Additional information: H (273K) -20 kcal/mol, H; (273K) = -15 kcal/mol, HE(273K) = -41 kcal/mol C. - C - 15 cal/mol K Cc = 30 cal/mol K k=0.01 dm at 300 K E - 10000 cal/mol mols PFR (a) Plot and then analyze the conversion and temperature as a function of PFR volume up to where X-0.85. Describe the trends. (b) What is the maximum inlet temperature one could have so that the boiling point of the liquid (550 K) would not be exceeded even for complete conversion? ni
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


