Question: please explain 3. Shown is a molecule in its acidic (fully protonated form). At what pH value could the structure exist as it is shown
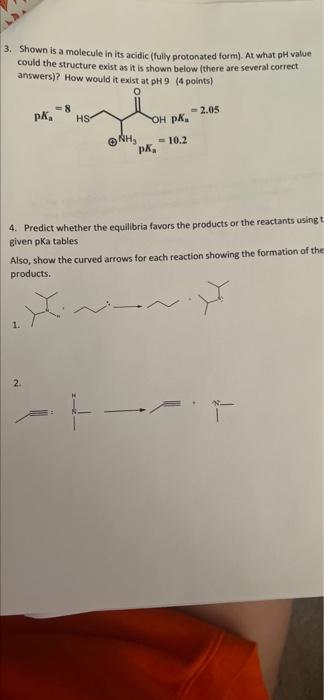
3. Shown is a molecule in its acidic (fully protonated form). At what pH value could the structure exist as it is shown below (there are several correct answers)? How would it exist at pH 9 (4 points) =8 PK HS 2.05 OH OK. = 10.2 NH . 4. Predict whether the equilibria favors the products or the reactants using given pka tables Also, show the curved arrows for each reaction showing the formation of the products
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


