Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #1 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #1!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEWTO CHEMISTRY! I AM A COMPLETE NEWBIE!
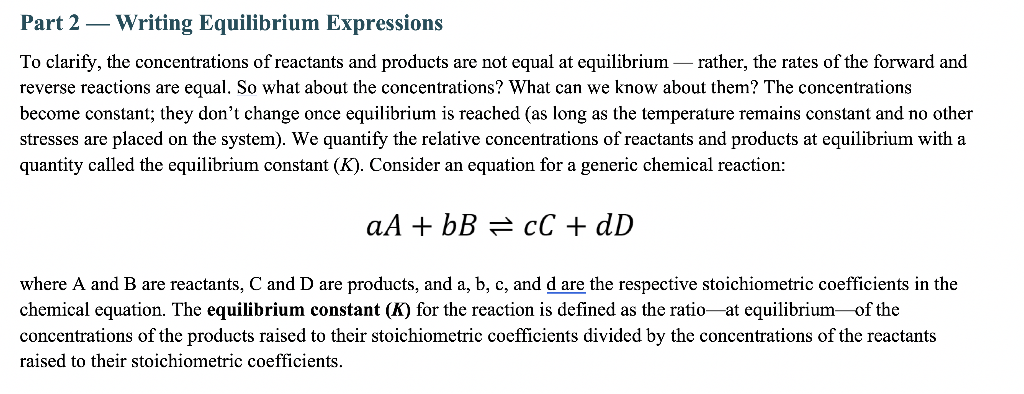
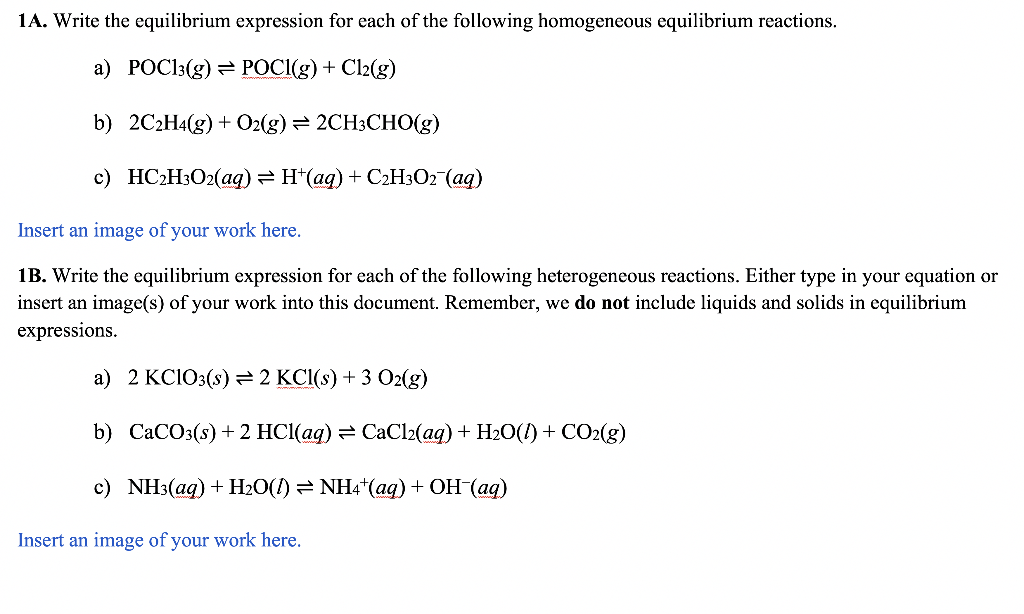
Part 2 - Writing Equilibrium Expressions To clarify, the concentrations of reactants and products are not equal at equilibrium - rather, the rates of the forward and reverse reactions are equal. So what about the concentrations? What can we know about them? The concentrations become constant; they don't change once equilibrium is reached (as long as the temperature remains constant and no other stresses are placed on the system). We quantify the relative concentrations of reactants and products at equilibrium with a quantity called the equilibrium constant (K). Consider an equation for a generic chemical reaction: aA+bBcC+dD where A and B are reactants, C and D are products, and a,b,c, and d are the respective stoichiometric coefficients in the chemical equation. The equilibrium constant (K) for the reaction is defined as the ratio-at equilibrium-of the concentrations of the products raised to their stoichiometric coefficients divided by the concentrations of the reactants raised to their stoichiometric coefficients. 1A. Write the equilibrium expression for each of the following homogeneous equilibrium reactions. a) POCl3(g)POCl(g)+Cl2(g) b) 2C2H4(g)+O2(g)2CH3CHO(g) c) HC2H3O2(aq)H+(aq)+C2H3O2(aq) Insert an image of your work here. 1B. Write the equilibrium expression for each of the following heterogeneous reactions. Either type in your equation or insert an image(s) of your work into this document. Remember, we do not include liquids and solids in equilibrium expressions. a) 2KClO3(s)2KCl(s)+3O2(g) b) CaCO3(s)+2HCl(aq)CaCl2(aq)+H2O(l)+CO2(g) c) NH3(aq)+H2O(l)NH4+(aq)+OH(aq) Insert an image of your work here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


