Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #1 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #1!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
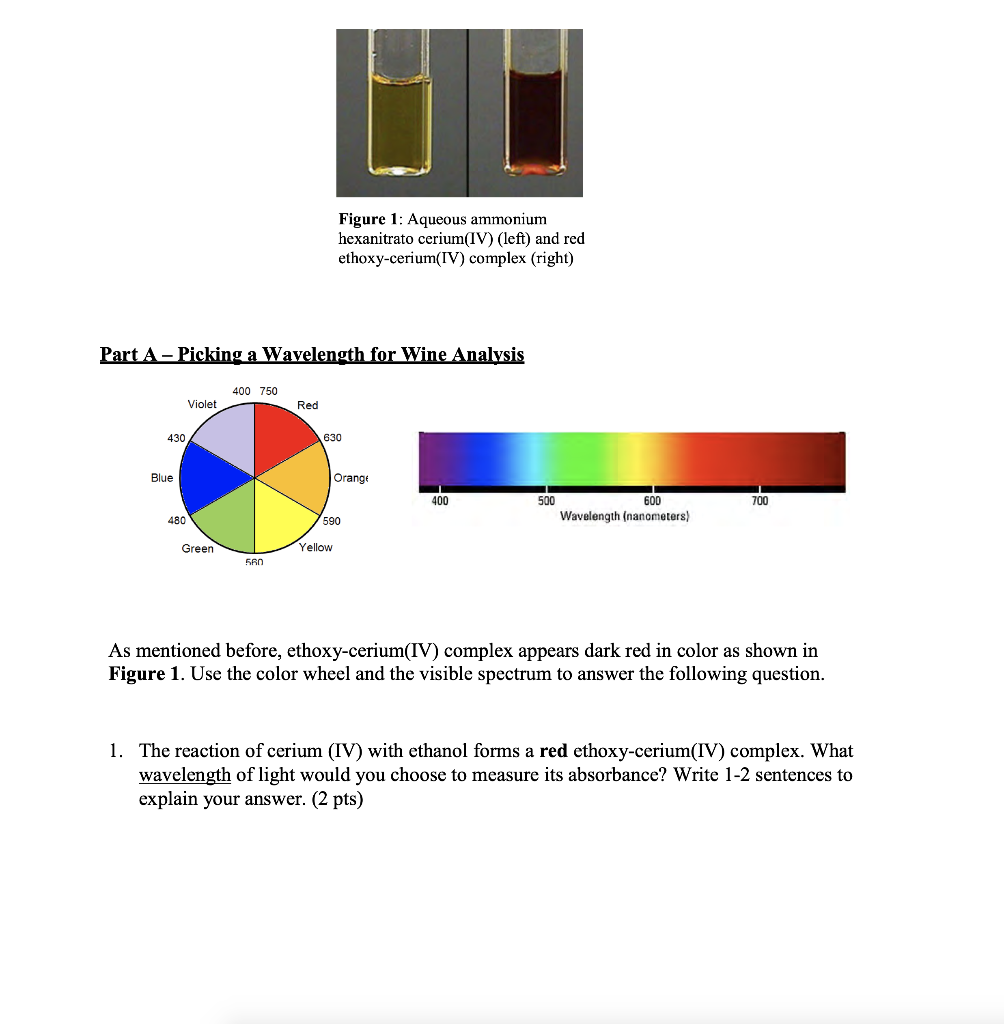
Figure 1: Aqueous ammonium hexanitrato cerium(IV) (left) and red ethoxy-cerium(IV) complex (right) Part A - Picking a Wavelength for Wine Analysis 400 750 Violet Red 430 630 Blue Orange 400 700 500 600 Wavelength (nanometers) 480 590 Green Yellow 560 As mentioned before, ethoxy-cerium(IV) complex appears dark red in color as shown in Figure 1. Use the color wheel and the visible spectrum to answer the following question. 1. The reaction of cerium (IV) with ethanol forms a red ethoxy-cerium(IV) complex. What wavelength of light would you choose to measure its absorbance? Write 1-2 sentences to explain your answer. (2 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


