Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #1 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #1!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
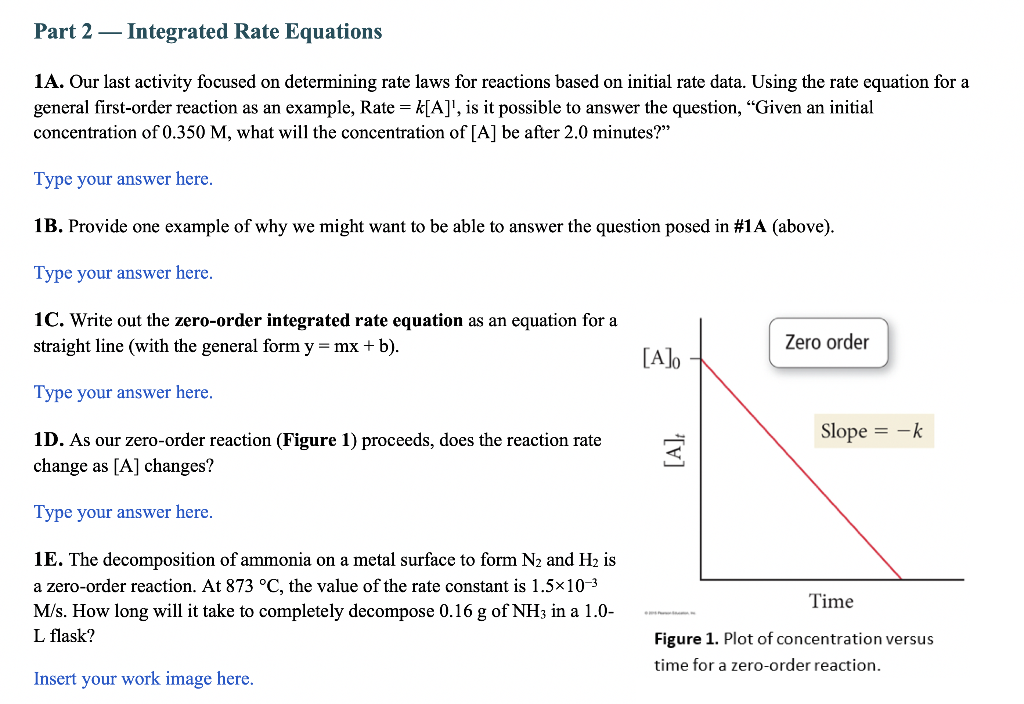
1A. Our last activity focused on determining rate laws for reactions based on initial rate data. Using the rate equation for a general first-order reaction as an example, Rate =k[A]1, is it possible to answer the question, "Given an initial concentration of 0.350M, what will the concentration of [A] be after 2.0 minutes?" Type your answer here. 1B. Provide one example of why we might want to be able to answer the question posed in \#1A (above). Type your answer here. 1C. Write out the zero-order integrated rate equation as an equation for a straight line (with the general form y=mx+b ). Type your answer here. 1D. As our zero-order reaction (Figure 1) proceeds, does the reaction rate change as [A] changes? Type your answer here. 1E. The decomposition of ammonia on a metal surface to form N2 and H2 is a zero-order reaction. At 873C, the value of the rate constant is 1.5103 M/s. How long will it take to completely decompose 0.16g of NH3 in a 1.0 L flask? Figure 1. Plot of concentration versus Insert your work image here. time for a zero-order reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


