Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #1 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #1!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
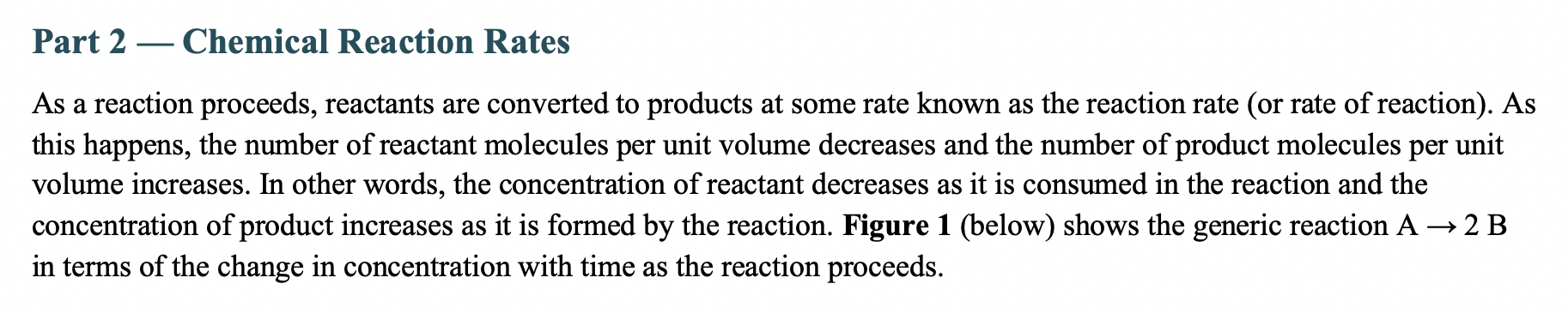
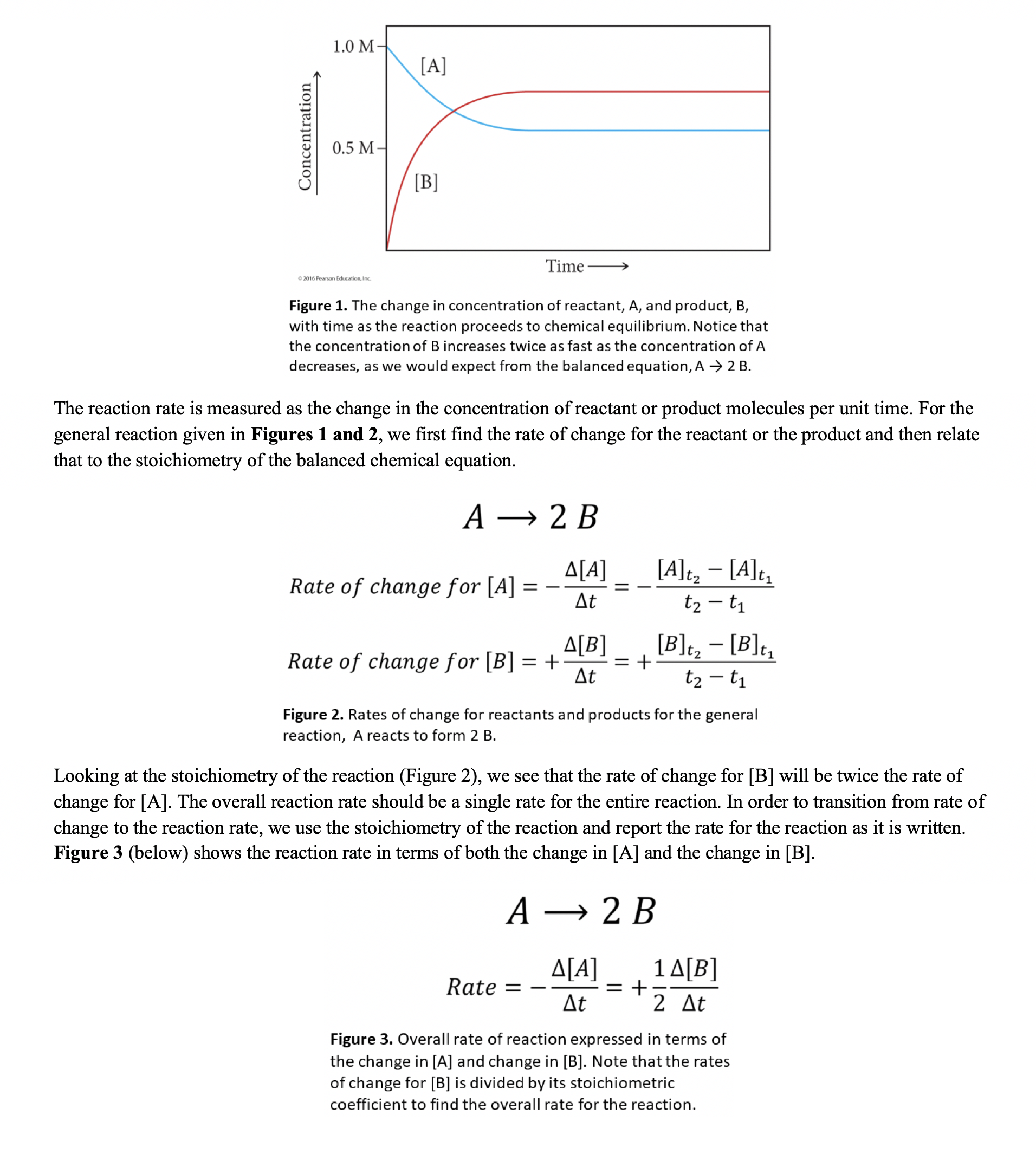

Part 2 - Chemical Reaction Rates As a reaction proceeds, reactants are converted to products at some rate known as the reaction rate (or rate of reaction). As this happens, the number of reactant molecules per unit volume decreases and the number of product molecules per unit volume increases. In other words, the concentration of reactant decreases as it is consumed in the reaction and the concentration of product increases as it is formed by the reaction. Figure 1 (below) shows the generic reaction A2B in terms of the change in concentration with time as the reaction proceeds. Figure 1. The change in concentration of reactant, A, and product, B, with time as the reaction proceeds to chemical equilibrium. Notice that the concentration of B increases twice as fast as the concentration of A decreases, as we would expect from the balanced equation, A2B. The reaction rate is measured as the change in the concentration of reactant or product molecules per unit time. For the general reaction given in Figures 1 and 2, we first find the rate of change for the reactant or the product and then relate that to the stoichiometry of the balanced chemical equation. A2BRateofchangefor[A]=t[A]=t2t1[A]t2[A]t1Rateofchangefor[B]=+t[B]=+t2t1[B]t2[B]t1 Figure 2. Rates of change for reactants and products for the general reaction, A reacts to form 2 B. Looking at the stoichiometry of the reaction (Figure 2), we see that the rate of change for [B] will be twice the rate of change for [A]. The overall reaction rate should be a single rate for the entire reaction. In order to transition from rate of change to the reaction rate, we use the stoichiometry of the reaction and report the rate for the reaction as it is written. Figure 3 (below) shows the reaction rate in terms of both the change in [A] and the change in [B]. A2BRate=t[A]=+21t[B] Figure 3. Overall rate of reaction expressed in terms of the change in [A] and change in [B]. Note that the rates of change for [B] is divided by its stoichiometric coefficient to find the overall rate for the reaction. For the reaction: 2A(g)+B(g)3C(g), answer the following: 1A. Determine the reaction rate for each of the reactants and products in terms of the change in concentration. Use the "Rate = " expression in Figure 3 to guide your answer. Insert an image of your equation here. 1B. When A is decreasing at a rate of 0.100M/s, how fast is B decreasing? Insert an image of your work here. 1C. When A is decreasing at a rate of 0.100M/s, how fast is C increasing? Insert an image of your work here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


