Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #9 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #9!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
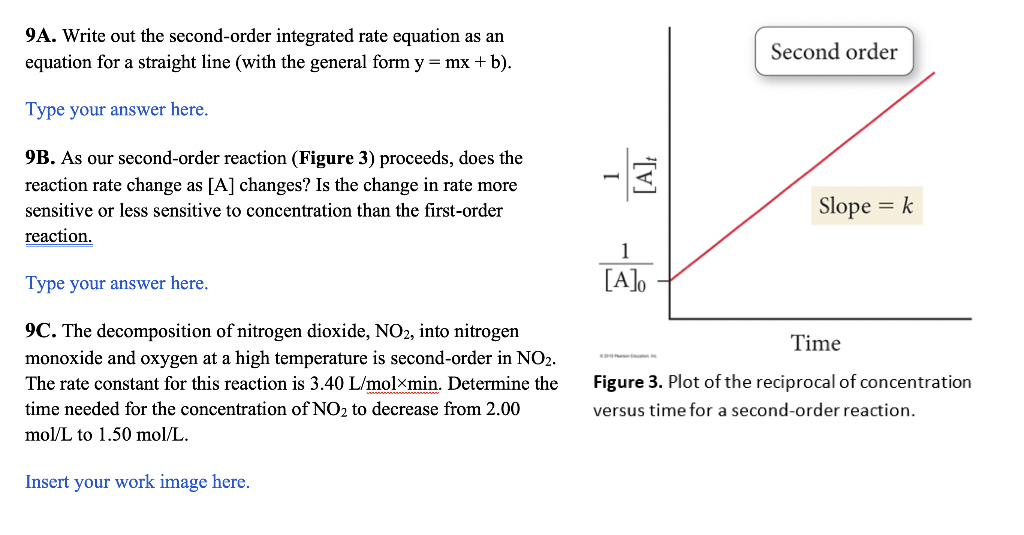
9A. Write out the second-order integrated rate equation as an equation for a straight line (with the general form y=mx+b ). 9B. As our second-order reaction (Figure 3 ) proceeds, does the reaction rate change as [A] changes? Is the change in rate more sensitive or less sensitive to concentration than the first-order reaction. Type your answer here. 9C. The decomposition of nitrogen dioxide, NO2, into nitrogen monoxide and oxygen at a high temperature is second-order in NO2. The rate constant for this reaction is 3.40L/molmin. Determine the Figure 3. Plot of the reciprocal of concentration time needed for the concentration of NO2 to decrease from 2.00 versus time for a second-order reaction. mol/L to 1.50mol/L. Insert your work image here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


