Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #4 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #4!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
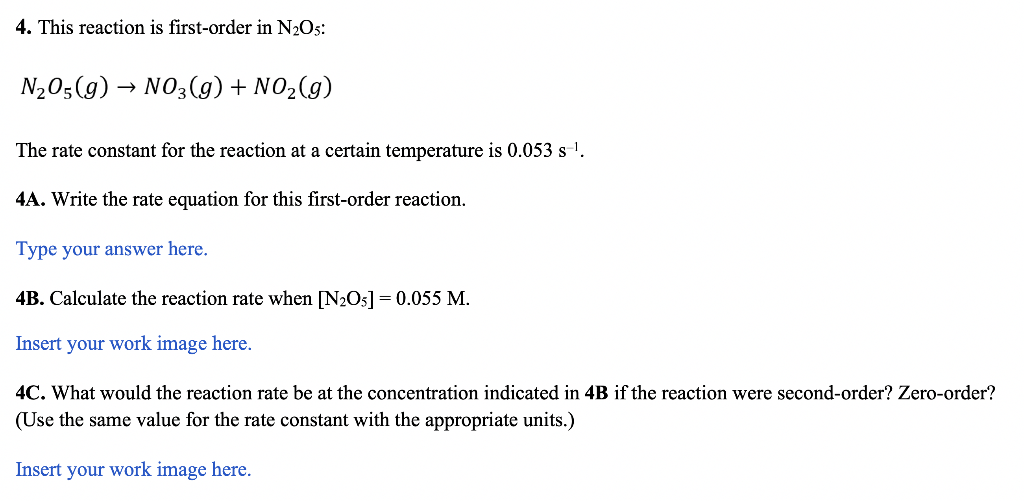
4. This reaction is first-order in N2O5 : N2O5(g)NO3(g)+NO2(g) The rate constant for the reaction at a certain temperature is 0.053s1. 4A. Write the rate equation for this first-order reaction. Type your answer here. 4B. Calculate the reaction rate when [N2O5]=0.055M. Insert your work image here. 4C. What would the reaction rate be at the concentration indicated in 4B if the reaction were second-order? Zero-order? (Use the same value for the rate constant with the appropriate units.) Insert your work image here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


