Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #2 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #2!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!

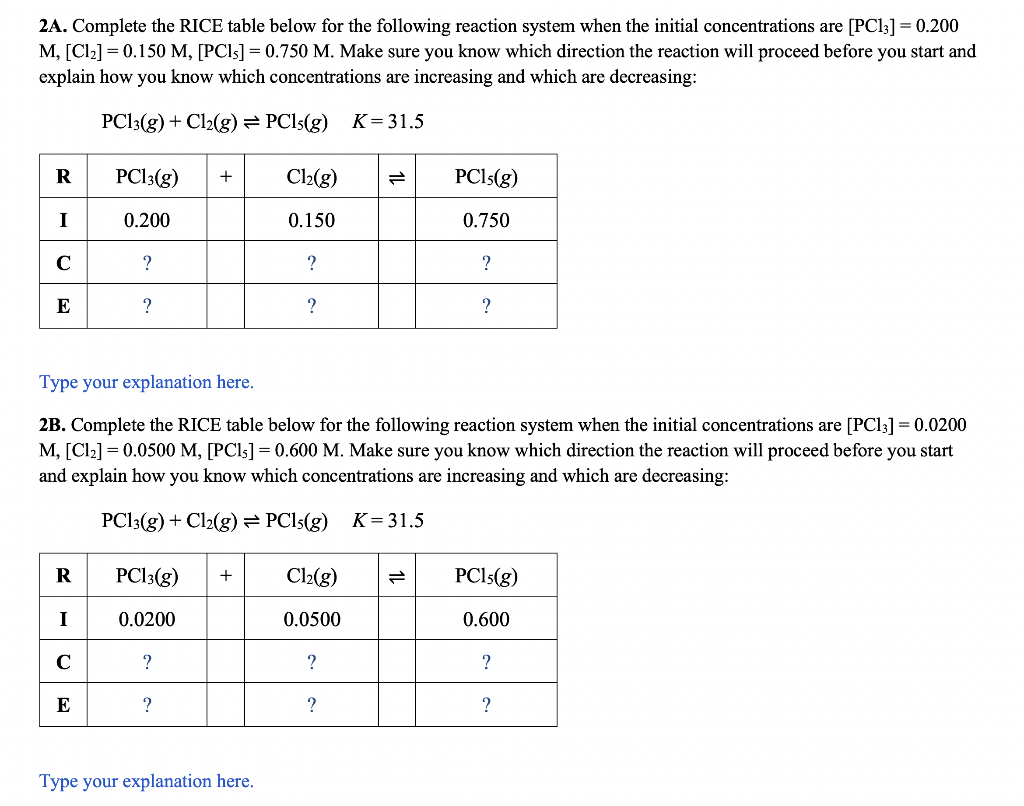
Part 1 - Solving More Complex Equilibrium Problems When solving equilibrium problems, it is important that we know which direction the reaction will run. This is often obvious from the problem statement as we often start only with reactants. Since there are no products, the reaction must run in the forward direction to establish equilibrium. However, sometimes we have initial concentrations of all of the reactants and products. In this case, we need to calculate Q and compare it to K to determine the direction of the reaction (forward or reverse). 2A. Complete the RICE table below for the following reaction system when the initial concentrations are [PCl3]=0.200 M,[Cl2]=0.150M,[PCl5]=0.750M. Make sure you know which direction the reaction will proceed before you start and explain how you know which concentrations are increasing and which are decreasing: PCl3(g)+Cl2(g)PCl5(g)K=31.5 Type your explanation here. 2B. Complete the RICE table below for the following reaction system when the initial concentrations are [PCl3]=0.0200 M,[Cl2]=0.0500M,[PCl5]=0.600M. Make sure you know which direction the reaction will proceed before you start and explain how you know which concentrations are increasing and which are decreasing: PCl3(g)+Cl2(g)PCl5(g)K=31.5 Type your explanation here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


