Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #5 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #5!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
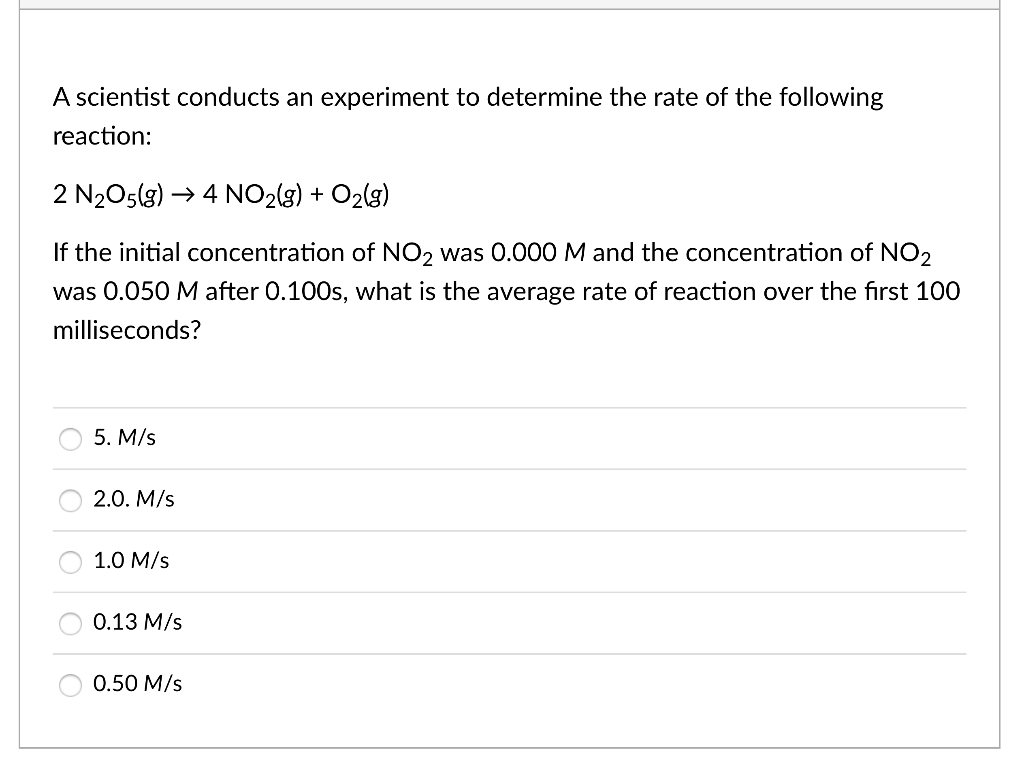
A scientist conducts an experiment to determine the rate of the following reaction: 2N2O5(g)4NO2(g)+O2(g) If the initial concentration of NO2 was 0.000M and the concentration of NO2 was 0.050M after 0.100s, what is the average rate of reaction over the first 100 milliseconds? 5.M/s 2.0.M/s 1.0M/s 0.13M/s 0.50M/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


