Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #1 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #1!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
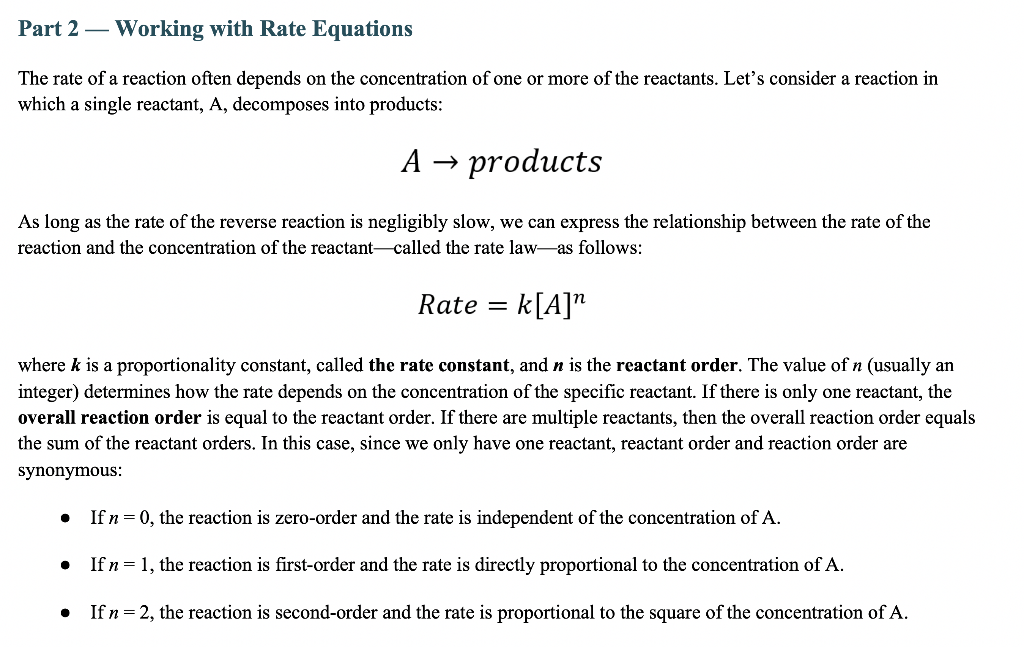
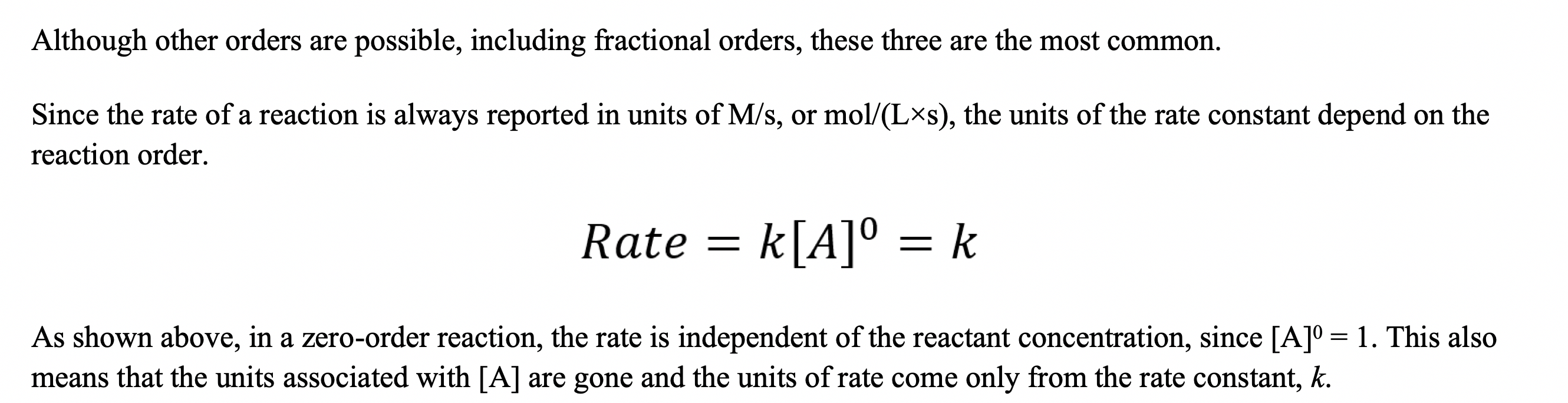

Part 2 - Working with Rate Equations The rate of a reaction often depends on the concentration of one or more of the reactants. Let's consider a reaction in which a single reactant, A, decomposes into products: Aproducts As long as the rate of the reverse reaction is negligibly slow, we can express the relationship between the rate of the reaction and the concentration of the reactant called the rate lawas follows: Rate=k[A]n where k is a proportionality constant, called the rate constant, and n is the reactant order. The value of n (usually an integer) determines how the rate depends on the concentration of the specific reactant. If there is only one reactant, the overall reaction order is equal to the reactant order. If there are multiple reactants, then the overall reaction order equal the sum of the reactant orders. In this case, since we only have one reactant, reactant order and reaction order are synonymous: - If n=0, the reaction is zero-order and the rate is independent of the concentration of A. - If n=1, the reaction is first-order and the rate is directly proportional to the concentration of A. - If n=2, the reaction is second-order and the rate is proportional to the square of the concentration of A. Although other orders are possible, including fractional orders, these three are the most common. Since the rate of a reaction is always reported in units of M/s, or mol/(Ls), the units of the rate constant depend on the reaction order. Rate=k[A]0=k As shown above, in a zero-order reaction, the rate is independent of the reactant concentration, since [A]0=1. This also means that the units associated with [A] are gone and the units of rate come only from the rate constant, k. Although other orders are possible, including fractional orders, these three are the most common. Since the rate of a reaction is always reported in units of M/s, or mol/(Ls), the units of the rate constant depend on the reaction order. Rate=k[A]0=k As shown above, in a zero-order reaction, the rate is independent of the reactant concentration, since [A]0=1. This also means that the units associated with [A] are gone and the units of rate come only from the rate constant, k. 1A. What are the units of k in a zero-order reaction? Type your answer here. In a first-order reaction, the rate is directly proportional to [A], since [A]1=[A]. Since the units of concentration come from [A], the units of k will be different for a first-order reaction than they were for a zero-order reaction. Rate=k[A]1 1B. What are the units for k in a first-order reaction? Type your answer here. In a second-order reaction, the rate is proportional to the square of [A]. That means that the units of k will be different once again. Both of the equations below are examples of second-order reactions. Note that in either case, we get units of concentration squared from our reactant concentrations, and k still needs to have units such that the rate is in units of M/s. Rate=k[A]2Rate=k[A]1[B]1 1C. What are the units for k in a second-order reaction? Type your answer here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


