Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #7 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #7!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEWTO CHEMISTRY! I AM A COMPLETE NEWBIE!
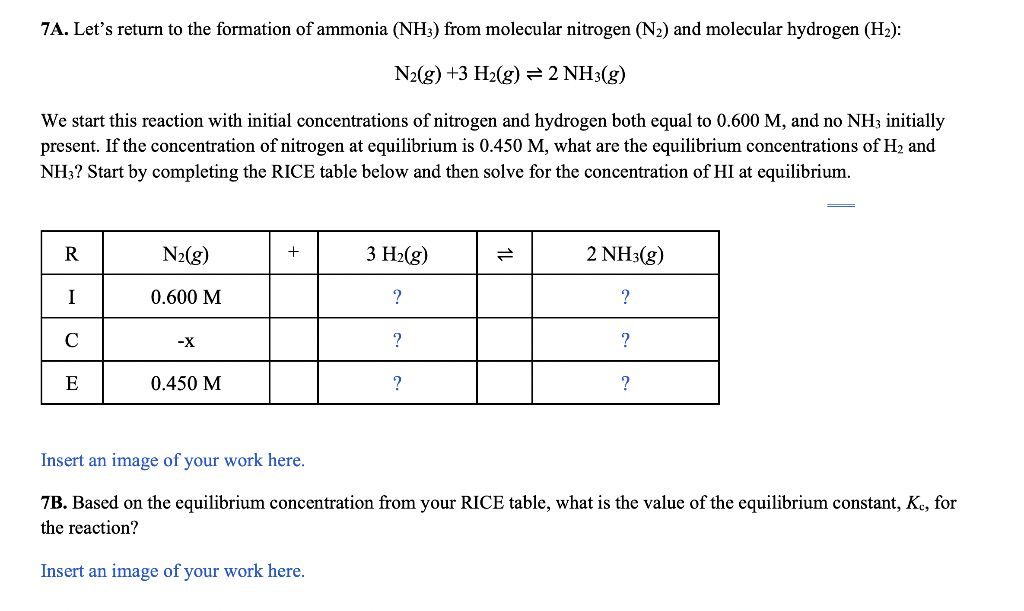
N2(g)+3H2(g)2NH3(g) We start this reaction with initial concentrations of nitrogen and hydrogen both equal to 0.600M, and no NH3 initially present. If the concentration of nitrogen at equilibrium is 0.450M, what are the equilibrium concentrations of H2 and NH3 ? Start by completing the RICE table below and then solve for the concentration of HI at equilibrium. Insert an image of your work here. 7B. Based on the equilibrium concentration from your RICE table, what is the value of the equilibrium constant, Kc, for the reaction? Insert an image of your work here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


