Question: Please explain how you solved, determining why the pair is more stable. Thank You Which cation/anion/radical in each of the following pairs is more stable?
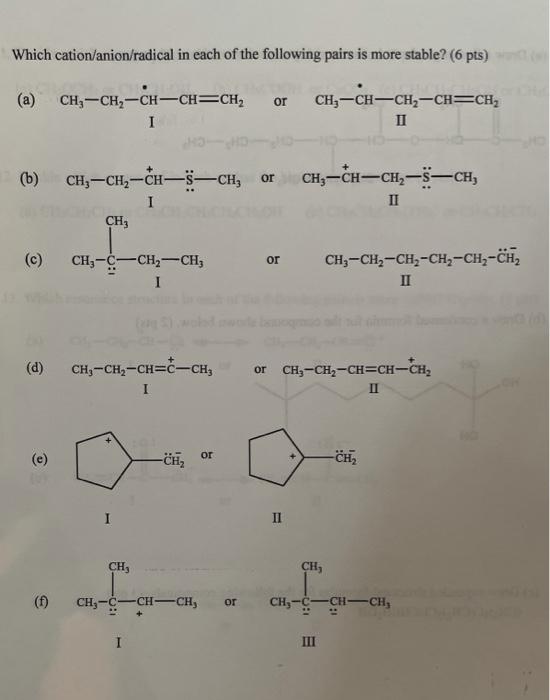
Which cation/anion/radical in each of the following pairs is more stable? (6 pts) or CH, -CH2-CH-CH=CH, I CH-CH=CH-CH=CH II (b) or CH3-CH-CH2-5-CH CH-CH2-CH-sCH, I CH, II (C) or CH - CH2 --CH I CH3-CH2-CH-CH2-CH2-CH, II (d) CH, -CH2-CH=C-CH, I or CH3-CH2-CH=CH-CH, II or -CH, -CH2 I II CH CH, 19- CH-C-CH-CH, or CH3-CH-CH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


