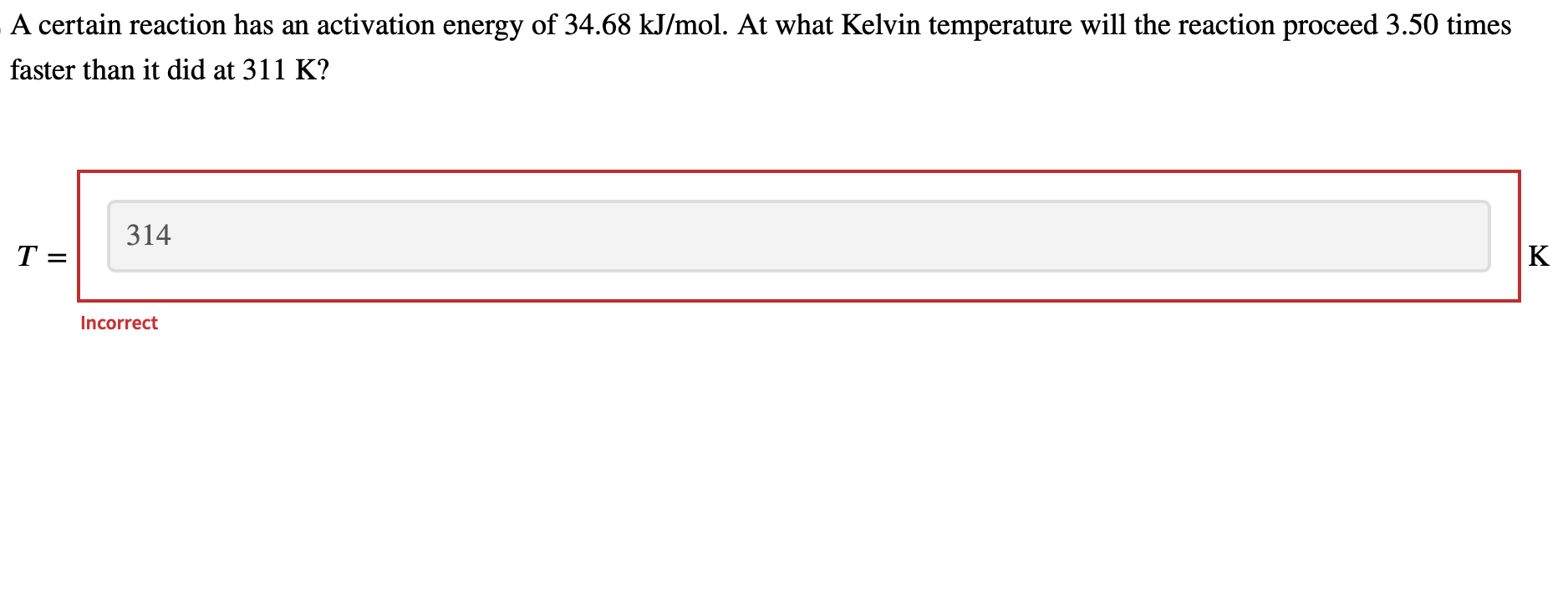
Question: please explain math clearly and show each step, thanks A certain reaction has an activation energy of 34.68kJ/mol. At what Kelvin temperature will the reaction
 please explain math clearly and show each step, thanks
please explain math clearly and show each step, thanks
A certain reaction has an activation energy of 34.68kJ/mol. At what Kelvin temperature will the reaction proceed 3.50 times faster than it did at 311K ? T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


