Question: PLEASE HELP ASAP. THIS IS A REACTION ENG PROBLEM a) Find the molar flow rates of Cl2, C3H5Cl, and C3H6Cl2, and the reactor temperature as
PLEASE HELP ASAP. THIS IS A REACTION ENG PROBLEM
a) Find the molar flow rates of Cl2, C3H5Cl, and C3H6Cl2, and the reactor temperature as a function of reactor length for the adiabatic operation.
b) Find the molar flow rates of Cl2, C3H5Cl, and C3H6Cl2, and the reactor temperature as a function of reactor length for constant wall temperature operation. What do you notice when you compare the extent of product formation and reactor temperature for both cases? why do you think this happens?
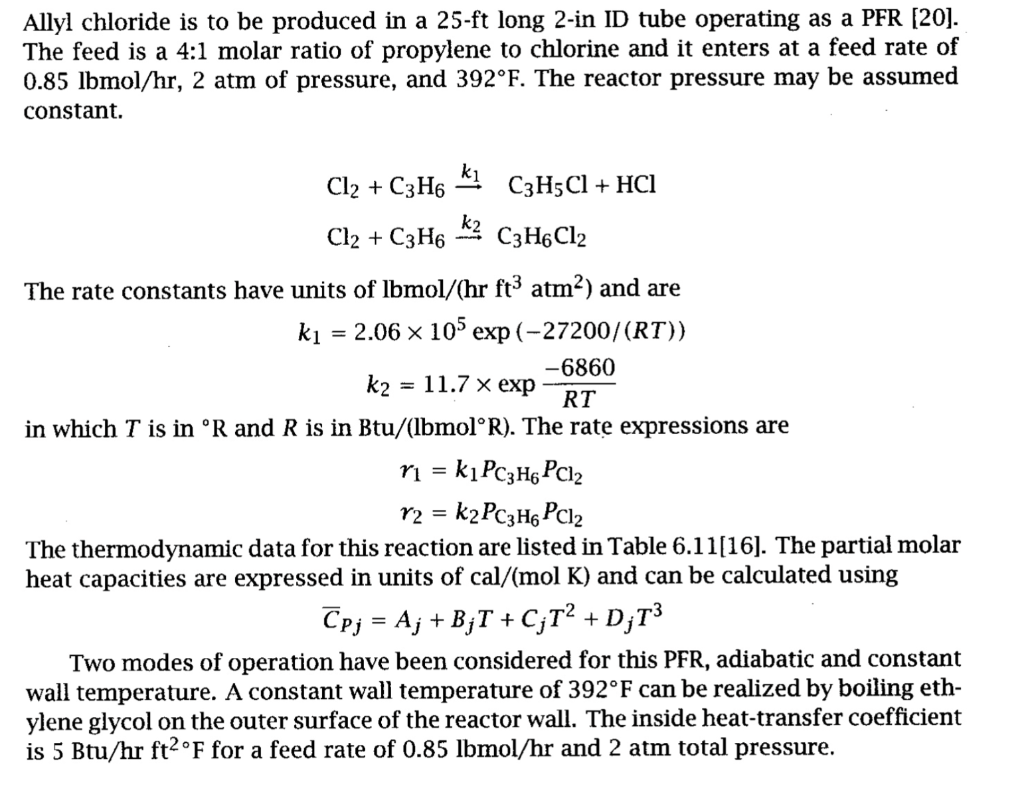
Allyl chloride is to be produced in a 25-ft long 2-in ID tube operating as a PFR [20]. The feed is a 4:1 molar ratio of propylene to chlorine and it enters at a feed rate of 0.85lbmol/hr,2atm of pressure, and 392F. The reactor pressure may be assumed constant. Cl2+C3H6k1C3H5Cl+HClCl2+C3H6k2C3H6Cl2 The rate constants have units of lbmol/(hrft3atm2) and are k1=2.06105exp(27200/(RT))k2=11.7expRT6860 in which T is in R and R is in Btu/(lbmolR). The rate expressions are r1=k1PC3H6PCl2r2=k2PC3H6PCl2 The thermodynamic data for this reaction are listed in Table 6.11[16]. The partial molar heat capacities are expressed in units of cal/(molK) and can be calculated using CPj=Aj+BjT+CjT2+DjT3 Two modes of operation have been considered for this PFR, adiabatic and constant wall temperature. A constant wall temperature of 392F can be realized by boiling ethylene glycol on the outer surface of the reactor wall. The inside heat-transfer coefficient is 5Btu/hrft2F for a feed rate of 0.85lbmol/hr and 2atm total pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


