Question: Please help! I will appreciate explanations to go with the answer. Amino acid used was glycine. Thank you. 2. Plot your data below to get
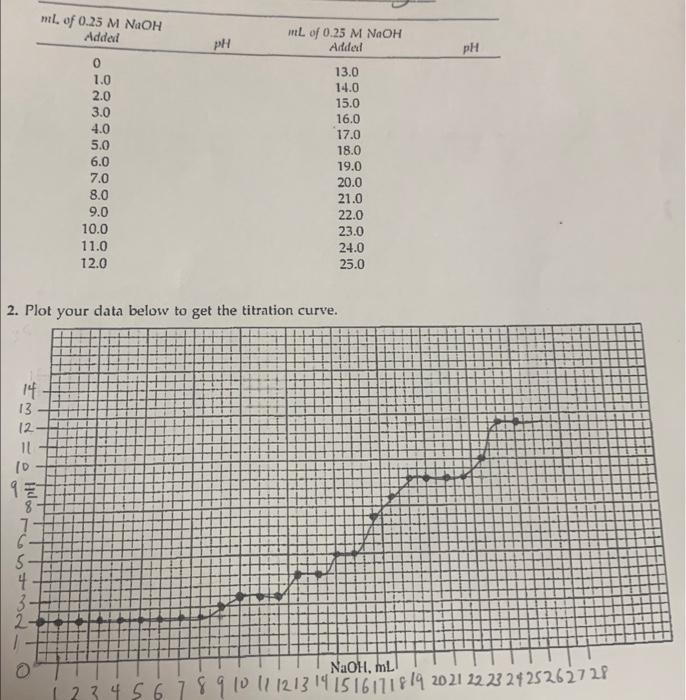
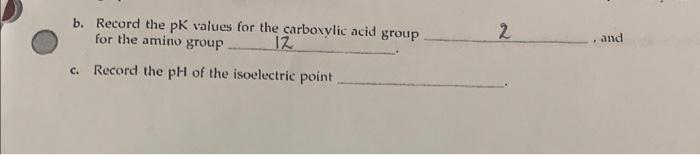

2. Plot your data below to get the titration curve. b. Record the pK values for the carboxylic acid group for the amino group 12 2 , and c. Record the pH of the isoelectric point 1. Look up the structure in your textbook of the amino acid you titrated. Write the structure you expect at pH (a) 2.5 and (b) 12 2. Compare your isoelectric point value (pl value) with the value of the amino acid listed in your textbook. How does it compare? Into which class does your amino acid fall? 3. Which data point can you obtain with greater accuracy from your graph-the pK values from the "legs" or the isoelectric point from the point of inflection? Explain your answer. 4. From the pKa value you obtained in your experiment, calculate the equilibrium constant, Ka. pKa=logKa 5. You are at the isoelectric point for a solution of leucine. If the number of negatively charged carboxylate groups, COO, is 11019, how many positively charged amino groups, NH3+, would there be
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


