Question: please help:( If you are unsure how to complete a slep of the question, assistance is avallable in Hints named by the step. Scroll down
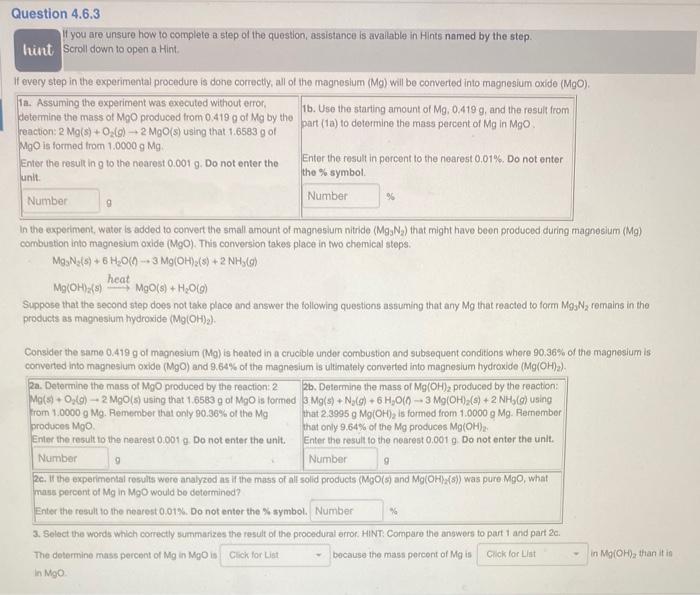
If you are unsure how to complete a slep of the question, assistance is avallable in Hints named by the step. Scroll down to open a Hint. If every step in the experimental procedure is done correctly, all of the magnesium (Mg) will be converted into magnesium oxide (MgO). In the experiment, water is added to convert the small amount of magneslum nitride (Mg3N2) that might have beon produced during magnesium (Mg) combustion into magnesium oxide (MgO). This conversion takes place in two chemical stops. Mg3N2(5)+6H2O()3Mg(OH)2(s)+2NH3(g)Mg(OH)(s)heatMgO(s)+H2O(g) Suppose that the second step does not take place and answer the following questions assuming that any Mg that reacted to form MgsN2 remains in the products as magnesium hydroxide (Mg(OH)2). Consider the same 0.419g of magnesium (Mg) is heated in a crucible under combustion and subsequent conditions where 90.36% of the magnesium is converted into magnesium oxide (MgO) and 9.64% of the magnesium is ultimately converted into magneslum hydroxide (Mg(OH)2 ). 3. Select the words which correctly summarizes the result of the procodural error. HiNT. Comparo the answers to part 1 and part 2c. The dotermine mass percent of Mg in MgO is bocause the mass porcont of Mg is in Mg(OH)2 than it in in NgO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


