Question: Please help me do these calculations. I have all the values I need, but I don't understand how to set up the equations with my
Please help me do these calculations. I have all the values I need, but I don't understand how to set up the equations with my values, and if my math is even right. The following information needed is below.
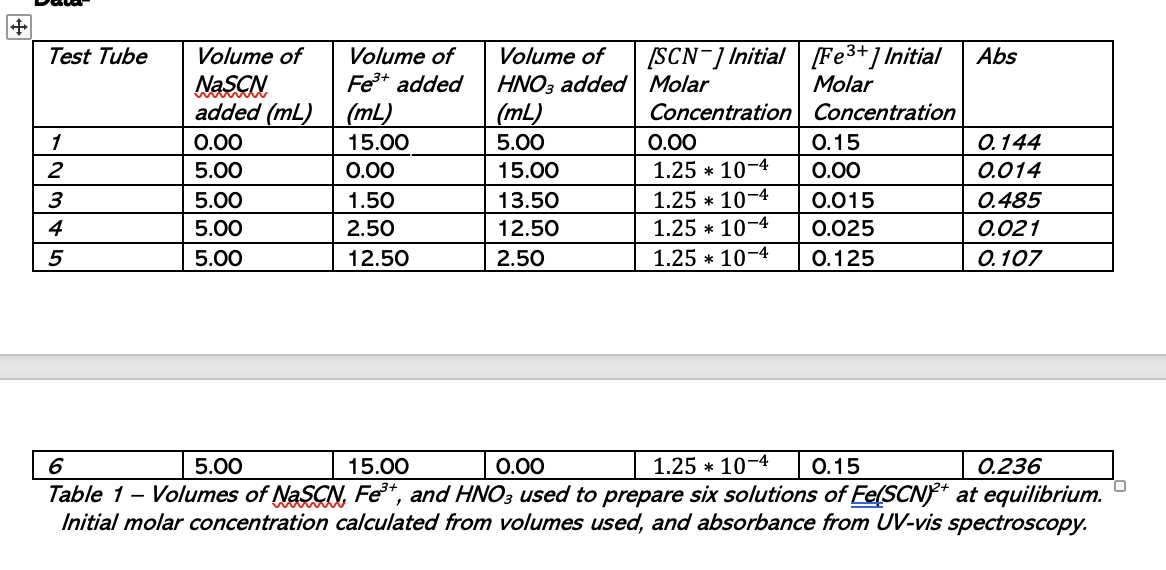
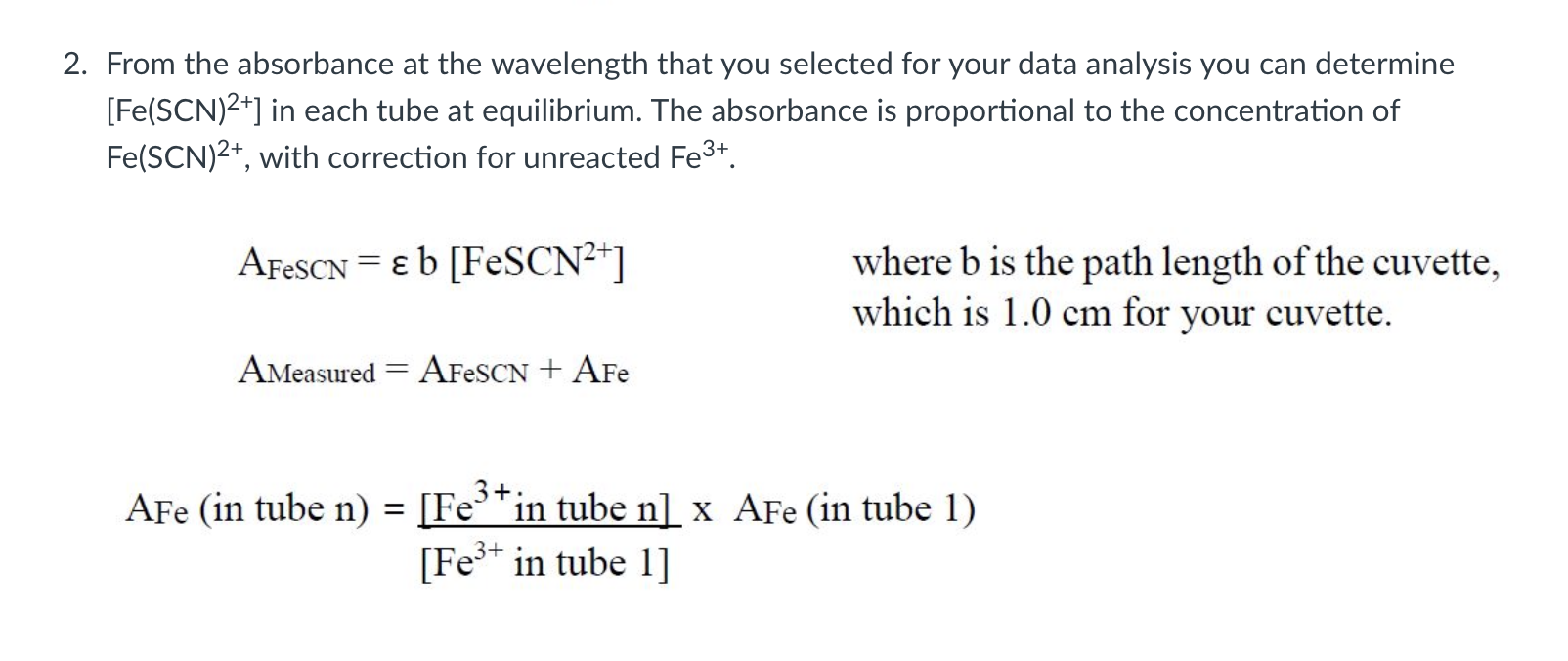
Table 1 - Volumes of NaSCN,Fe3+, and HNO3 used to prepare six solutions of Fe(SCN2+ at equilibrium. Initial molar concentration calculated from volumes used, and absorbance from UV-vis spectroscopy. 2. From the absorbance at the wavelength that you selected for your data analysis you can determine [Fe(SCN)2+] in each tube at equilibrium. The absorbance is proportional to the concentration of Fe(SCN)2+, with correction for unreacted Fe3+. AFeSCN=b[FeSCN2+] where b is the path length of the cuvette, which is 1.0cm for your cuvette. Ameasured=AFeSCN+AFe AFe( in tube n)=[Fe3+intube1][Fe3+intuben]AFe (in tube 1) Table 1 - Volumes of NaSCN,Fe3+, and HNO3 used to prepare six solutions of Fe(SCN2+ at equilibrium. Initial molar concentration calculated from volumes used, and absorbance from UV-vis spectroscopy. 2. From the absorbance at the wavelength that you selected for your data analysis you can determine [Fe(SCN)2+] in each tube at equilibrium. The absorbance is proportional to the concentration of Fe(SCN)2+, with correction for unreacted Fe3+. AFeSCN=b[FeSCN2+] where b is the path length of the cuvette, which is 1.0cm for your cuvette. Ameasured=AFeSCN+AFe AFe( in tube n)=[Fe3+intube1][Fe3+intuben]AFe (in tube 1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


