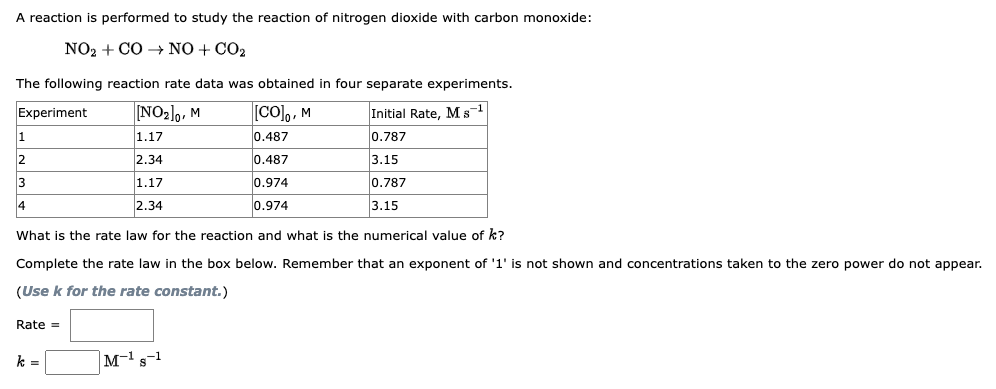
Question: Please help me figure this question out, I do not understand. Thank you in advance!! NO2+CONO+CO2 The following reaction rate data was obtained in four
Please help me figure this question out, I do not understand. Thank you in advance!!
NO2+CONO+CO2 The following reaction rate data was obtained in four separate experiments. What is the rate law for the reaction and what is the numerical value of k ? Complete the rate law in the box below. Remember that an exponent of ' 1 ' is not shown and concentrations taken to the zero power do (Use k for the rate constant.) Rate = k=M1s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


