Question: The heat capacity of hydrogen may be represented by Cpm = (1.554 + 0.00227) J K mol1 Calculate the change in entropy of the
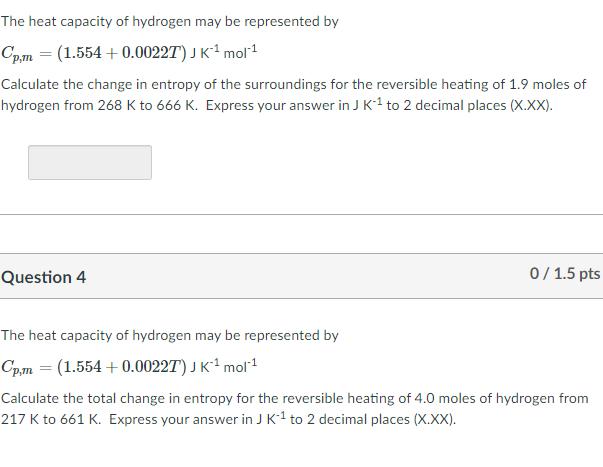
The heat capacity of hydrogen may be represented by Cpm = (1.554 + 0.00227) J K mol1 Calculate the change in entropy of the surroundings for the reversible heating of 1.9 moles of hydrogen from 268 K to 666 K. Express your answer in J K1 to 2 decimal places (X.XX). Question 4 0/ 1.5 pts The heat capacity of hydrogen may be represented by Cpm = (1.554 + 0.00227) JK mol Calculate the total change in entropy for the reversible heating of 4.0 moles of hydrogen from 217 K to 661 K. Express your answer in J K1 to 2 decimal places (X.XX).
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
3 cp 1554 00022T Jk moli for reversible adiabatic process AS sys CP Ti 2 po... View full answer

Get step-by-step solutions from verified subject matter experts


