Question: Need help with Part B please :) The reactant concentration in a zero-order reaction was 7.00102M after 155s and 1.50102M after 390s. What is the
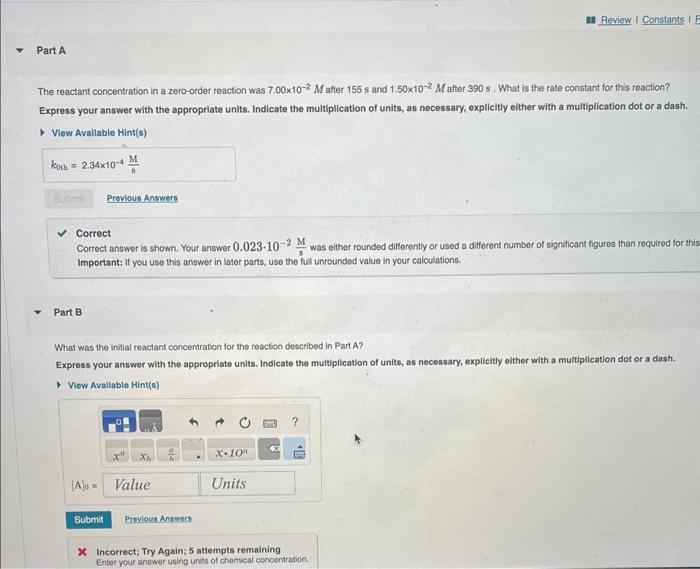
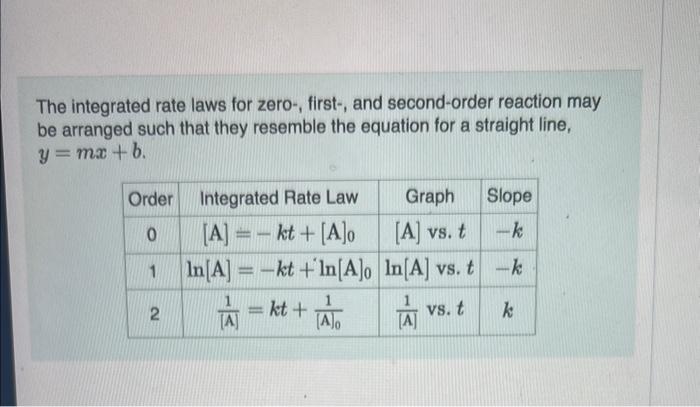
The reactant concentration in a zero-order reaction was 7.00102M after 155s and 1.50102M after 390s. What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. Correct Correct answer is shown. Your answer 0.023102sM was eithar rounded differently or used a differont number of signiticant figures than required for thi! Important: if you use this answer in later parts, use the ful unrounded value in your calculations. Part B What was the initial reactant concentration for the reaction described in Part A? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly elther with a multiplication dot or a dash. x Incorrect; Try Again; 5 attempts remaining Entor your answer using units of chomical conconiration. The integrated rate laws for zero-, first-, and second-order reaction may be arranged such that they resemble the equation for a straight line, y=mx+b
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


