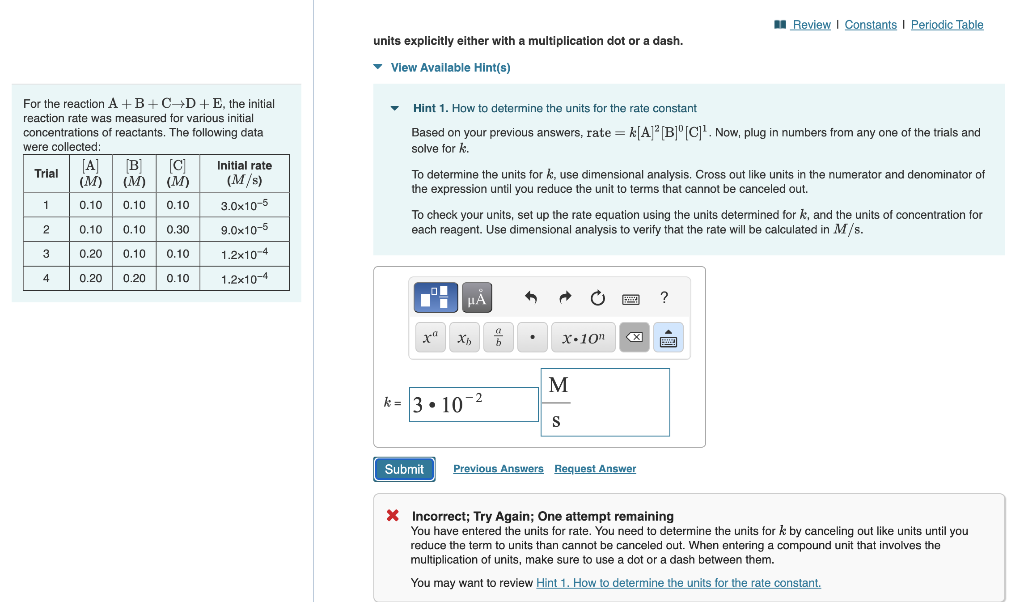
Question: please help. units explicitly either with a multiplication dot or a dash. View Available Hint(s) For the reaction A+B+CD+E, the initial reaction rate was measured
 please help.
please help.
units explicitly either with a multiplication dot or a dash. View Available Hint(s) For the reaction A+B+CD+E, the initial reaction rate was measured for various initial Hint 1. How to determine the units for the rate constant concentrations of reactants. The following data Based on your previous answers, rate =k[A]2[B]0[C]1. Now, plug in numbers from any one of the trials and solve for k. To determine the units for k, use dimensional analysis. Cross out like units in the numerator and denominator of the expression until you reduce the unit to terms that cannot be canceled out. To check your units, set up the rate equation using the units determined for k, and the units of concentration for each reagent. Use dimensional analysis to verify that the rate will be calculated in M/s. * Incorrect; Try Again; One attempt remaining You have entered the units for rate. You need to determine the units for k by canceling out like units until you reduce the term to units than cannot be canceled out. When entering a compound unit that involves the multiplication of units, make sure to use a dot or a dash between them. You may want to review Hint 1. How to determine the units for the rate constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


