Question: please please help! The integrated rate laws for zero-, first-, and second-order reaction may be arranged such that they resemble the equation for a straight
![Law Graph 0 [A] =- kt +[A]o [A] vs. t 1 In[A]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f853956cdcc_35766f85395162e7.jpg)
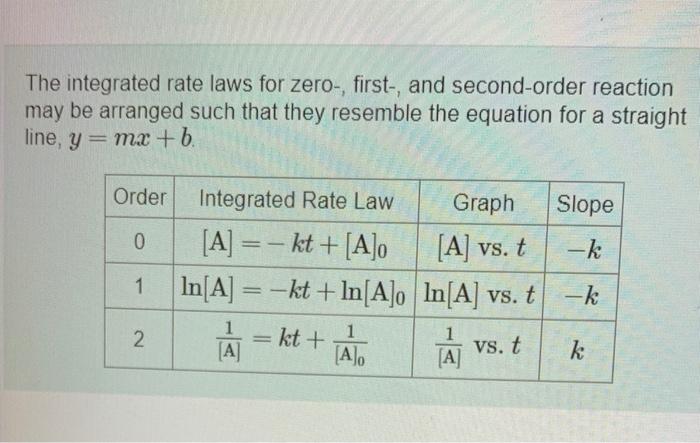
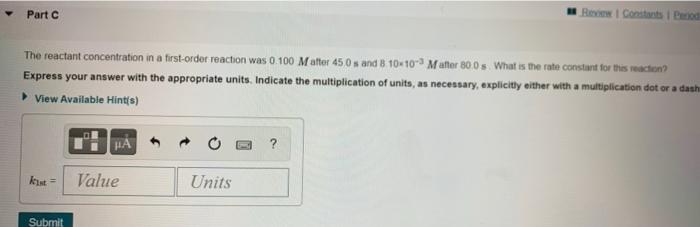
The integrated rate laws for zero-, first-, and second-order reaction may be arranged such that they resemble the equation for a straight line, y = mx + b Slope -k Order Integrated Rate Law Graph 0 [A] =- kt +[A]o [A] vs. t 1 In[A] = -kt + In[A]o In[A] vs. t 1 1 1 [A] [A [A] -k N kt + vs. t k Part A The reactant concentration in a zero-ordot reaction was 0 100 M after 165 s and 2.00x102 Mattor 330 s What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. View Available Hints) HA kuh Value Units Submit Part B Complete previous partes) Part The reactant concentration in a first-order reaction was 0.100 Matter 450s and 8 10x10- Matter 800 s What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a danh View Available Hints) Part Row i Constantino The reactant concentration in a first-order reaction was 0.100 Matter 450 s and 8 10-10-) Matter 800 s What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash View Available Hints) ? kust Value Units Submit Part The reactant concentration in a second order reaction was 0.550 Matter 205 s and 1.00x10-2 Matter 35 What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication datora dash. View Available Hints) ? kad Value Units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


