Question: please help! will upvote! thank you so much for eveything :) Sthalic acid (H2C8H4O4) is a diprotic acid with Kal=1.12103 and Ka2=3.90106. Determine he pH
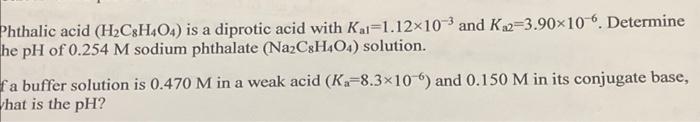
Sthalic acid (H2C8H4O4) is a diprotic acid with Kal=1.12103 and Ka2=3.90106. Determine he pH of 0.254M sodium phthalate (Na2C8H4O4) solution. a buffer solution is 0.470M in a weak acid (Ka=8.3106) and 0.150M in its conjugate base, hat is the pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


