Question: PLEASE HELP WITH 2-4 clear photos Experimentel Dial setting Volume V. (ml) 391 Temp T. (0) Pressure Contar? P.) 1052 Pressure vs Temperature (C) 0
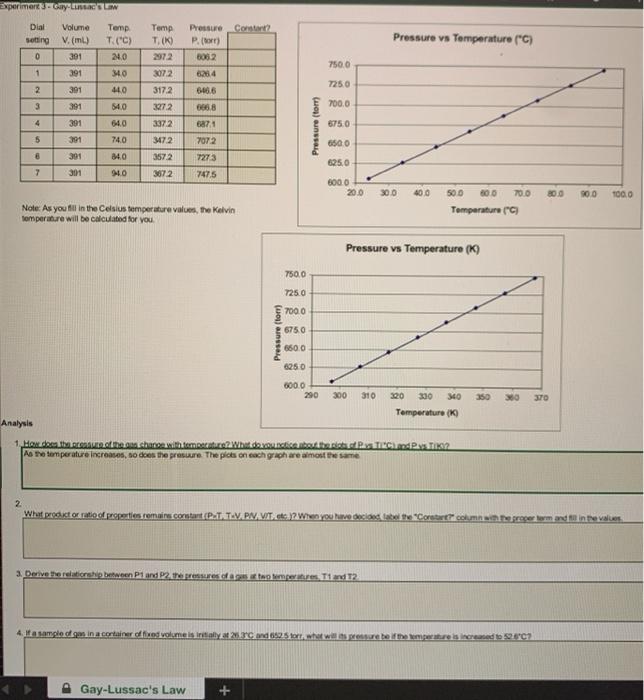
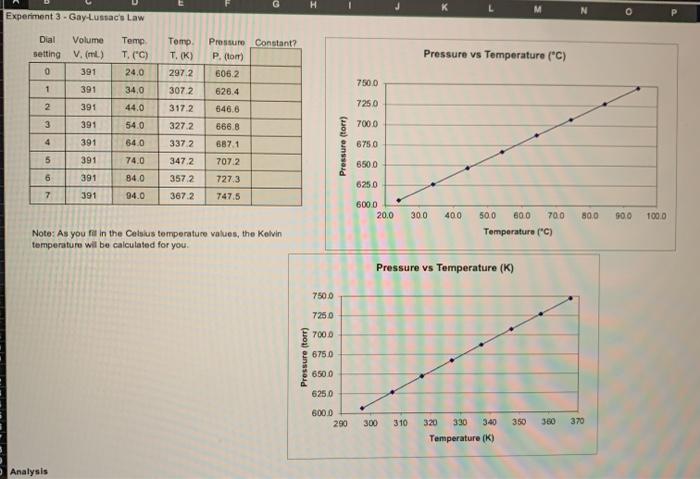

Experimentel Dial setting Volume V. (ml) 391 Temp T. (0) Pressure Contar? P.) 1052 Pressure vs Temperature (C) 0 Temp T (C) 24.0 340 440 54,0 7500 1 391 3072 317.2 2 7250 391 3 391 327.2 700.0 4 391 64.0 3372 6284 646.6 6858 6871 7072 7273 7475 Pressure (tom) 6750 6500 5 391 74.0 391 6 7 240 0 3172 3572 372 625.0 301 6000 200 300 40.0 70.0 500 1000 Note: As you fill in the Celsius temperature values, the Kelvin temperature will be calculated for you 500 600 Temperatura Pressure vs Temperature (9 750.0 7250 7000 Pressure (tom) 6750 650.0 6250 000.0 290 300 310 350 370 340 Temperature ( Analysis 1. How does the share with me? What do you TPS o the temperature increases, so does the presure. The picts on each grach are almost the same 2 What protocol properties remains.com (PTAT VPNAVIT. When you have decided the Constant column opererm and finale 3. Derve the relationship between P1 and 22. redagstogeland 12 4 ita samples in a container dig volumes intalya 2330 and 0.2 shot wit is pressure be the operature is created to 20 Gay-Lussac's Law G Experiment 3 Gay Lussac's Law Dial Volume setting V. (m) 0 391 1 391 Temp T. (C) 24.0 Tomp , T. (K) 2972 Pressure vs Temperature (*C) Pressure Constant? P.com 6062 626.4 646.6 7500 3072 34.0 44.0 2 391 7250 3 391 540 3172 327.2 3372 666.8 7000 4 391 6871 707.2 6750 Pressure (tor) 5 391 391 64.0 74.0 84.0 94.0 6500 347.2 3572 6 727.3 747.5 6250 7 391 367.2 6000 20.0 30.0 400 80.0 900 100.0 500 600 700 Temperatura (c) Note: As you fill in the Celsius temperature values, the Kolvin temperature will be calculated for you. Pressure vs Temperature (K) 7500 7250 700.0 Pressure torr) 6750 6500 6250 6000 290 300 310 350 380 370 320 330 340 Temperature (K) Analysis 2. What product or ratio of properties remains constant (PXT, TXV.PN, VIT, etc.)? When you have decided, label the "Constant?" column with the proper term and fall in the values 3. Derive the relationship between P1 and P2, the pressures of a gas at two temperatures, T1 and T2 4. If a sample of gas in a container of fixed volume is initially at 26.3C and 652.5 to what will pressure be the temperature is increased to 52.6'C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


