Question: please help with this question Silver iodide crystallizes in the zinc blende structure. The separation between nearest neighbor cations and anions is approximately 325pm and
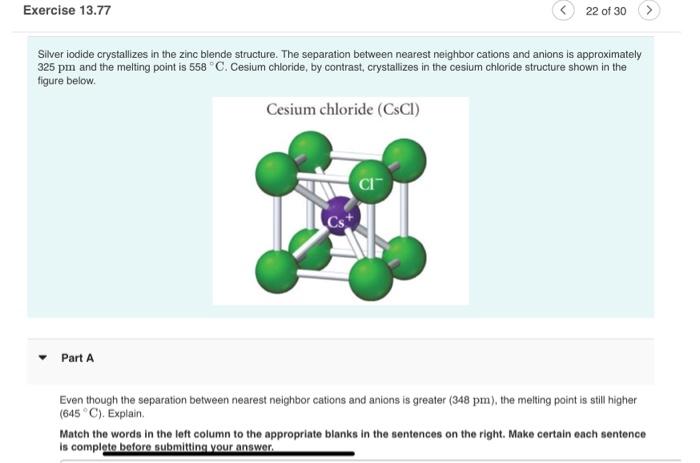
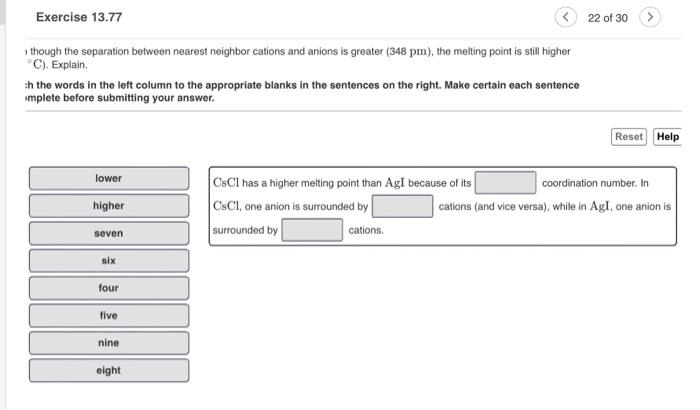
Silver iodide crystallizes in the zinc blende structure. The separation between nearest neighbor cations and anions is approximately 325pm and the melting point is 558C. Cesium chloride, by contrast, crystallizes in the cesium chloride structure shown in the figure below. Part A Even though the separation between nearest neighbor cations and anions is greater ( 348 pm), the melting point is still higher (645C). Explain. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. I though the separation between nearest neighbor cations and anions is greater ( 348pm ), the melting point is still higher C). Explain. :h the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence implete before submitting your answer. CsCl has a higher melting point than AgI because of its coordination number. In CsCl, one anion is surrounded by cations (and vice versa), while in AgI, one anion is surrounded by cations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


