Question: please help...use same number..!.! 3. Mass balance with reaction and separation: Consider the process to make ammonia from gaseous hydrogen and nitrogen shown in the
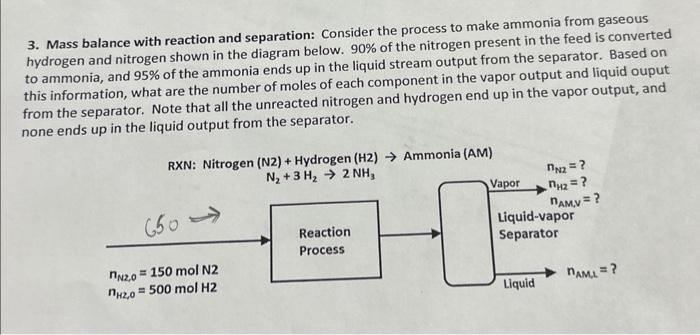
3. Mass balance with reaction and separation: Consider the process to make ammonia from gaseous hydrogen and nitrogen shown in the diagram below. 90% of the nitrogen present in the feed is converted to ammonia, and 95% of the ammonia ends up in the liquid stream output from the separator. Based on this information, what are the number of moles of each component in the vapor output and liquid ouput from the separator. Note that all the unreacted nitrogen and hydrogen end up in the vapor output, and none ends up in the liquid output from the separator
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


