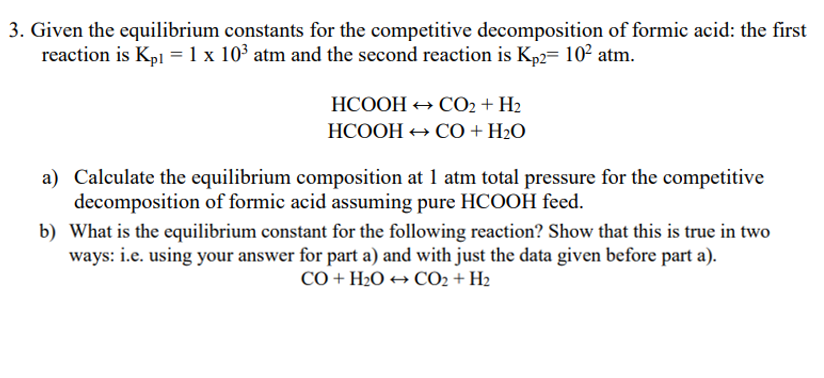
Question: please show 2 ways for part b Given the equilibrium constants for the competitive decomposition of formic acid: the first reaction is Kp1=1103atm and the
please show 2 ways for part b
Given the equilibrium constants for the competitive decomposition of formic acid: the first reaction is Kp1=1103atm and the second reaction is Kp2=102atm. HCOOHCO2+H2HCOOHCO+H2O a) Calculate the equilibrium composition at 1atm total pressure for the competitive decomposition of formic acid assuming pure HCOOH feed. b) What is the equilibrium constant for the following reaction? Show that this is true in two ways: i.e. using your answer for part a) and with just the data given before part a). CO+H2OCO2+H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


