Question: Please show all work and explain your steps if possible. I will give a like if the solution is correct! 4. Saturated steam. Saturated steam
Please show all work and explain your steps if possible. I will give a like if the solution is correct!
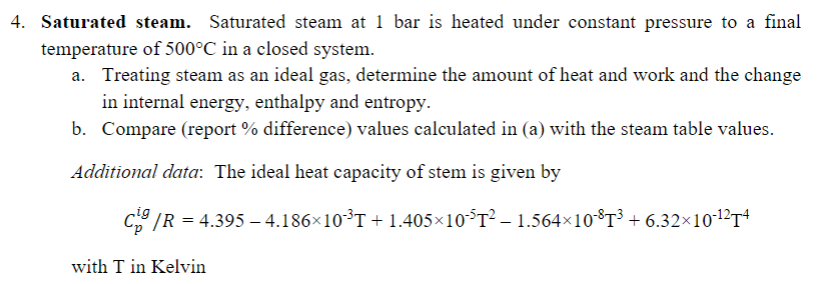
4. Saturated steam. Saturated steam at 1 bar is heated under constant pressure to a final temperature of 500C in a closed system. a. Treating steam as an ideal gas, determine the amount of heat and work and the change in internal energy, enthalpy and entropy. b. Compare (report % difference) values calculated in (a) with the steam table values. Additional data: The ideal heat capacity of stem is given by c. /R = 4.395 4.186x10T + 1.405x10$T- 1.564x10-T3 + 6.32x10-1214 ig with T in Kelvin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


