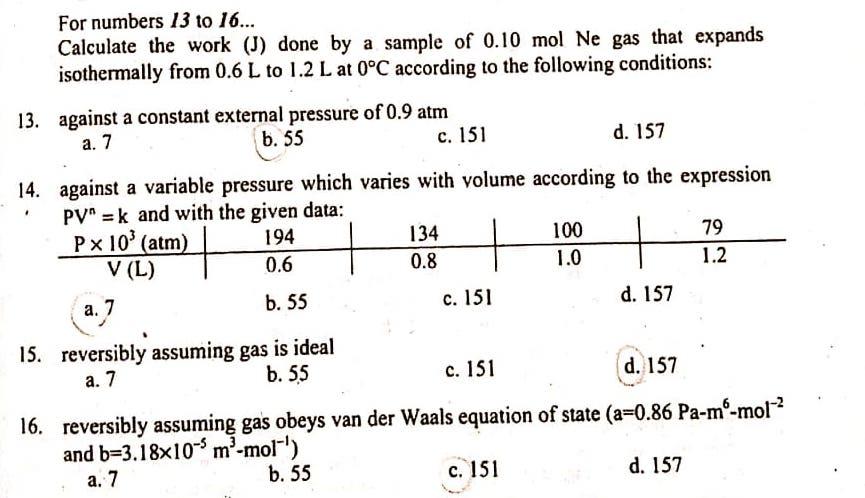
Question: Please show complete solution... thank you! For numbers 13 to 16... Calculate the work (J) done by a sample of 0.10 mol Ne gas that
 Please show complete solution... thank you!
Please show complete solution... thank you!
For numbers 13 to 16... Calculate the work (J) done by a sample of 0.10 mol Ne gas that expands isothermally from 0.6 L to 1.2 L at 0C according to the following conditions: 13. against a constant external pressure of 0.9 atm a. 7 b. 55 b c. 151 d. 157 . 14. against a variable pressure which varies with volume according to the expression PV = k and with the given data: Px 10' (atm) 194 134 100 79 V (L) 0.6 0.8 1.0 1.2 b. 55 d. 157 c. 151 a. 7 15. reversibly assuming gas is ideal b. 55 c. 151 d. 157 a. 7 16. reversibly assuming gas obeys van der Waals equation of state (a=0.86 Pa-m-mol-? and b=3.18x10-m'-mol-') b. 55 c. 151 d. 157 a. 7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


