Question: please show steps for b and c . thank you An aqueous NaCl solution is made using 120g of Part B NaCi diuted to a
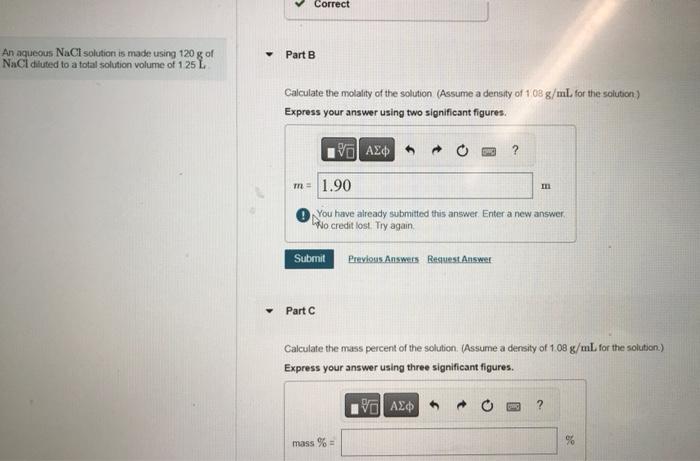
An aqueous NaCl solution is made using 120g of Part B NaCi diuted to a total solution volume of 1:25 L Calculate the molality of the solution (Assume a density of 1.08g/mL, for the soluticn) Express your answer using two significant figures. (1) You have already submitted this answer. Enter a new answer. Wo credit lost. Try again. Part C Calculate the mass percent of the solution. (Assume a density of 1.08g/mL for the solution) Express your answer using three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


