Question: Please show work for both buffers! Part B Buffer 1 pH of 0.1m6.57.5 pH of 0.01m7.79 pH of 0.001M7.65 Buffer 2 pH of 0.1m6.5pH of
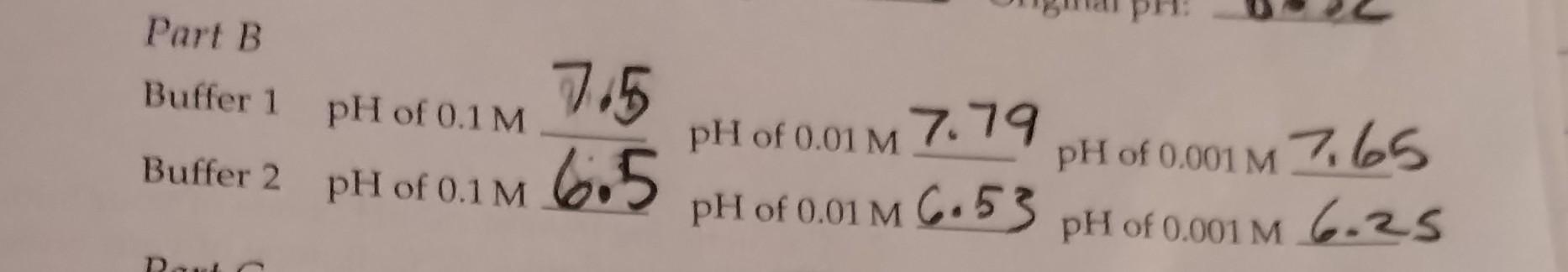

Please show work for both buffers!
Part B Buffer 1 pH of 0.1m6.57.5 pH of 0.01m7.79 pH of 0.001M7.65 Buffer 2 pH of 0.1m6.5pH of 0.01m6.53pH of 0.001m6.25 2. Calculate the theoretical pH of one of your buffers at 0C. Assume that room temperature is 22C. If none of your buffers is listed on the table of changing pKa with temperature, do this problem for TRIS at pH8.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


