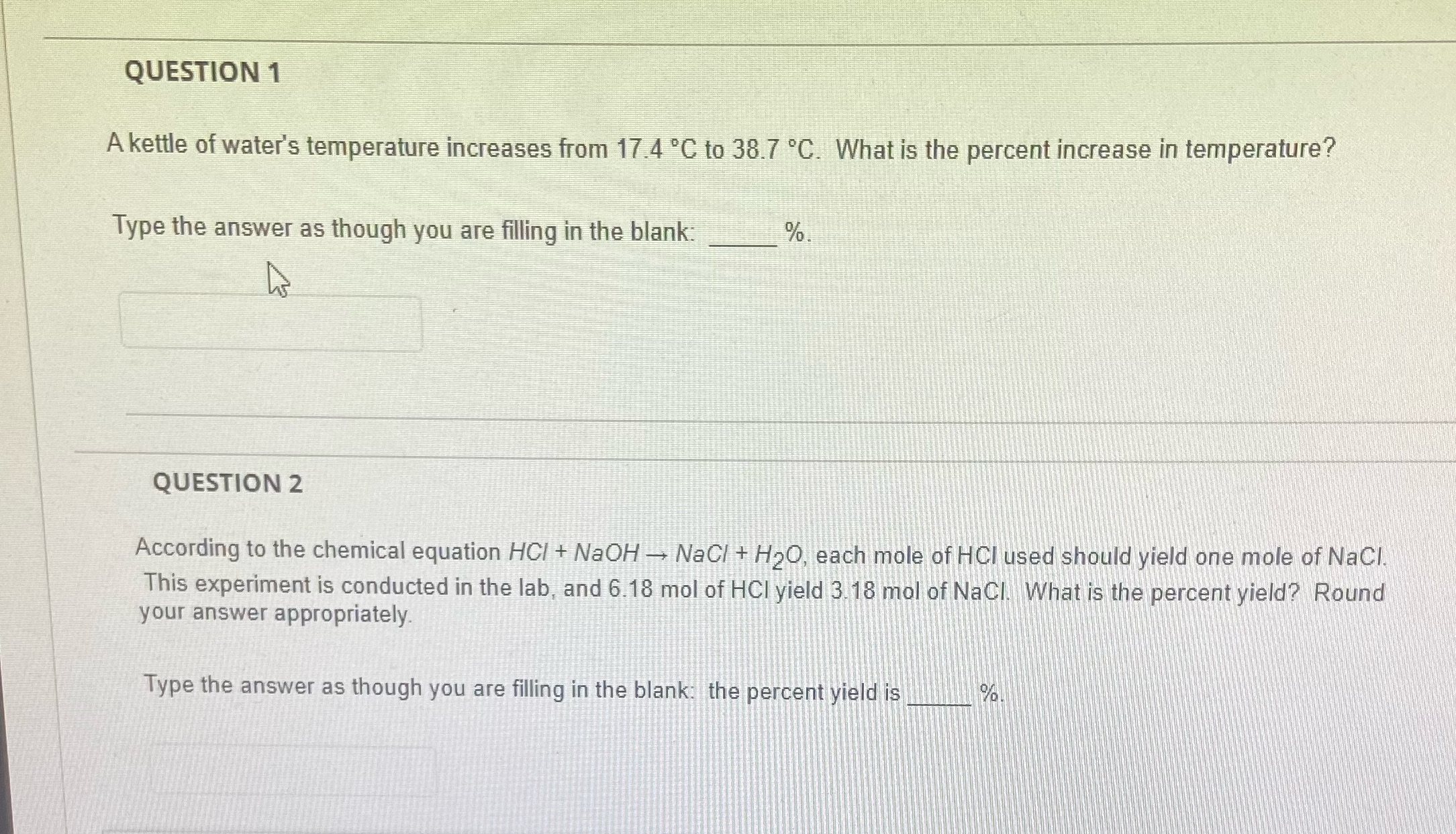
Question: Please solve both questions question 1 and 2 both QUESTION 1 A kettle of water's temperature increases from 17.4 'C to 38.7 .C. What is
Please solve both questions question 1 and 2 both
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


