Question: Please solve complete i will give you upvote Q20) Assuming complete combustion. For the following fuels, estimate their carbon intensity (in kg of C/ MJ
Please solve complete i will give you upvote
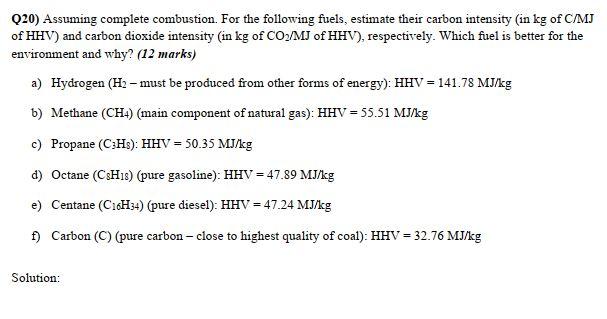
Q20) Assuming complete combustion. For the following fuels, estimate their carbon intensity (in kg of C/ MJ of HHV) and carbon dioxide intensity (in kg of CO/MJ of HHV), respectively. Which fuel is better for the environment and why? (12 marks) a) Hydrogen (H2 must be produced from other forms of energy): HHV = 141.78 MJ/kg b) Methane (CH4) (main component of natural gas): HHV = 55.51 MJ/kg c) Propane (CH3): HHV = 50.35 MJ/kg d) Octane (CsHis) (pure gasoline): HHV = 47.89 MJ/kg e) Centane (C16H34) (pure diesel): HHV = 47.24 MJ/kg f) Carbon (C) (pure carbon - close to highest quality of coal): HHV = 32.76 MJ/kg Solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


