Question: Please solve the questions which is not solved Please solve the questions which is not solved Please solve the questions which is not solved A
Please solve the questions which is not solved
Please solve the questions which is not solved
Please solve the questions which is not solved
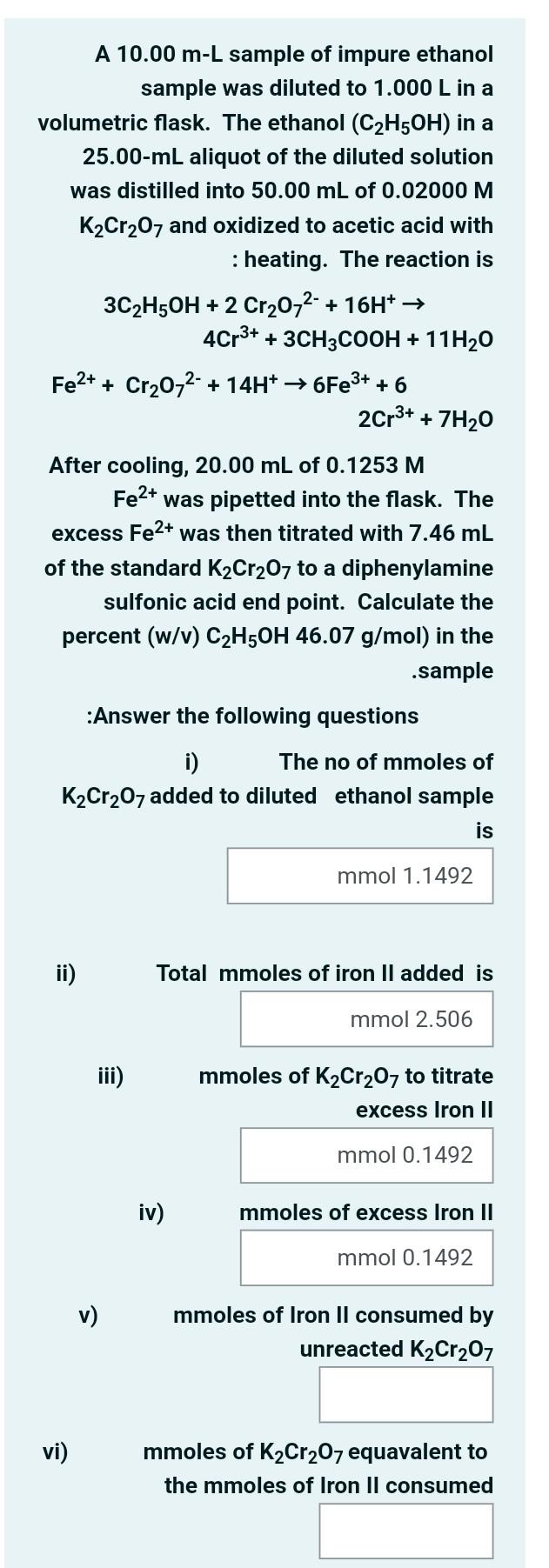
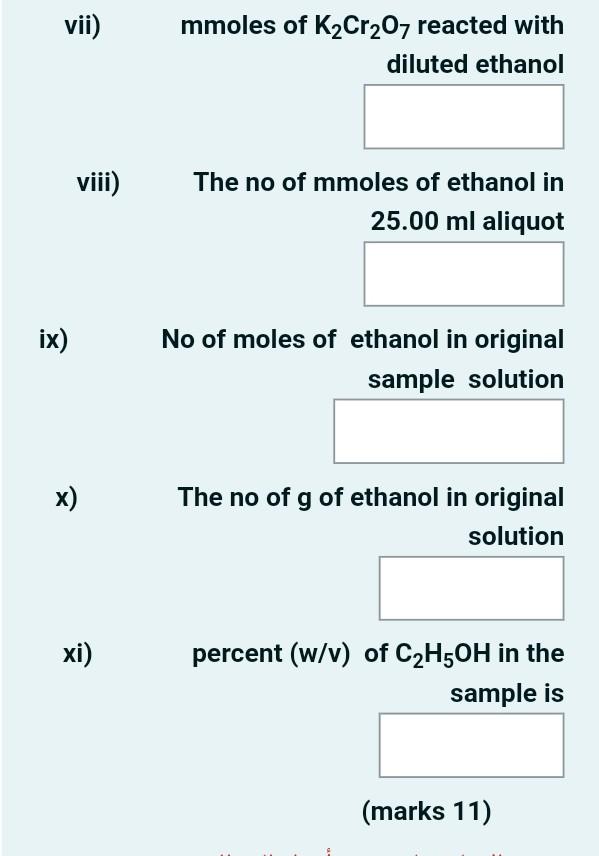
A 10.00 m-L sample of impure ethanol sample was diluted to 1.000 L in a volumetric flask. The ethanol (C2H5OH) in a 25.00-ml aliquot of the diluted solution was distilled into 50.00 mL of 0.02000 M K2Cr207 and oxidized to acetic acid with :heating. The reaction is 3C2H5OH + 2 Cr2O72- + 16H+ 4Cr3+ + 3CH3COOH + 11H20 Fe2+ + Cr2O72- + 14H+ +6Fe3+ + 6 2Cr3+ + 7H20 After cooling, 20.00 mL of 0.1253 M Fe2+ was pipetted into the flask. The excess Fe2+ was then titrated with 7.46 mL of the standard K2Cr207 to a diphenylamine sulfonic acid end point. Calculate the percent (w/v) C2H5OH 46.07 g/mol) in the .sample :Answer the following questions i) The no of mmoles of K2Cr2O7 added to diluted ethanol sample is mmol 1.1492 ii) Total mmoles of iron Il added is mmol 2.506 iii) mmoles of K2Cr2O7 to titrate excess Iron II mmol 0.1492 iv) mmoles of excess Iron II mmol 0.1492 v) mmoles of Iron Il consumed by unreacted K2Cr207 vi) mmoles of K2Cr207 equavalent to the mmoles of Iron Il consumed vii) mmoles of K2Cr207 reacted with diluted ethanol viii) The no of mmoles of ethanol in 25.00 ml aliquot ix) No of moles of ethanol in original sample solution x) The no of g of ethanol in original solution xi) percent (w/v) of C2H5OH in the sample is (marks 11)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


