Question: Please solve this question for me based on these data 2.4 A liquidmixture of benzene-toluene is to be distilled in a fractionating tower at 101.3

Please solve this question for me based on these data
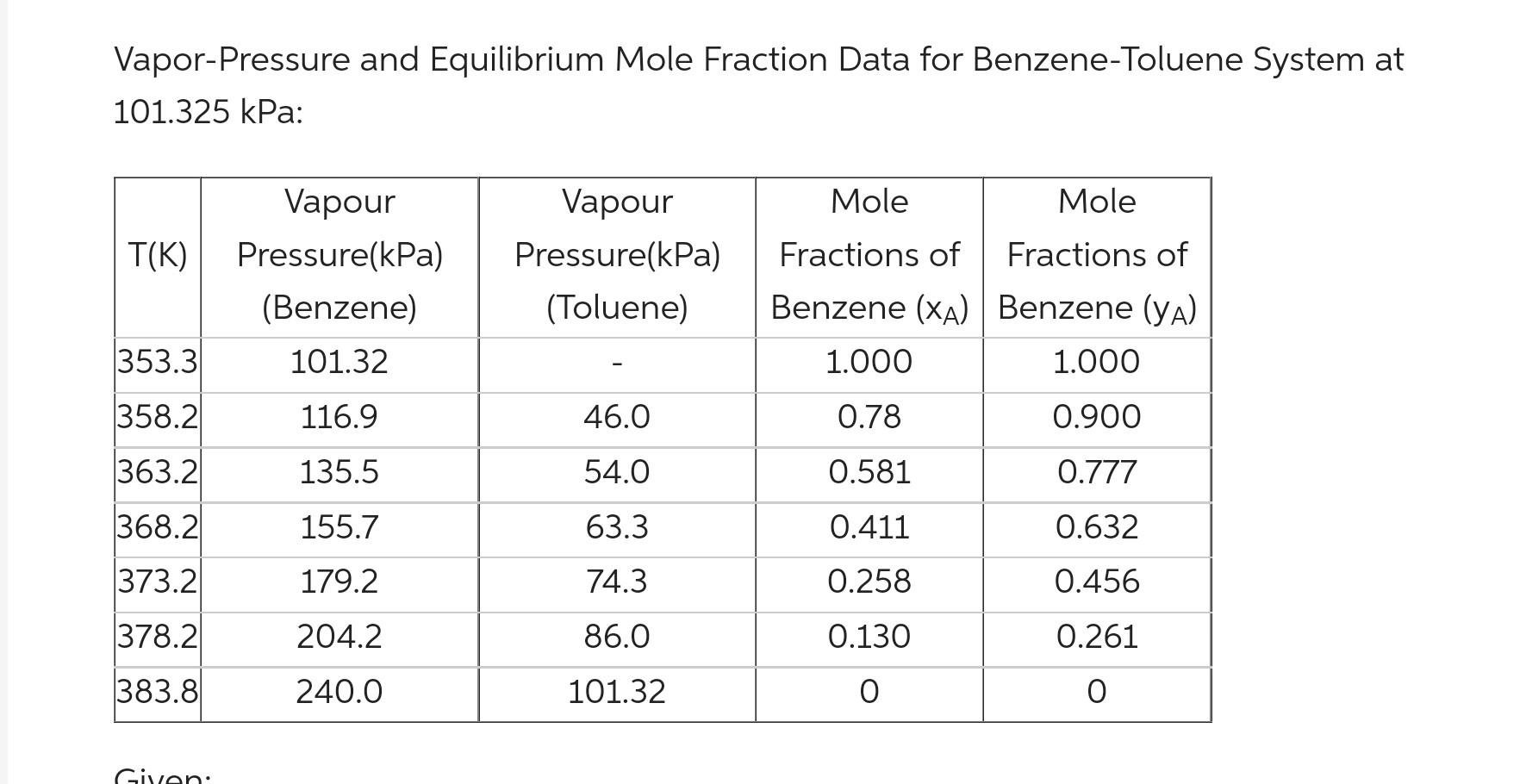
2.4 A liquidmixture of benzene-toluene is to be distilled in a fractionating tower at 101.3 KPa pressure. The feed of 100kmol/h is liquid containing 45 mol\% benzene and 55 mol \% toluene and entels at 327.6K. A distillate containing 95mol% benzene and a bottom containing 10 mol benzene. The reflux ratio is 4:1. The average heat capacity of feed is 159KJ/KmolmoK and The latent heat is 32099kJ/Kmol. The boiling point of the feed is 366.7K. Using the attached equilibrium data detemine: (1) The distildate and bottoms in Kmol/h. (2) The number of theoretical trays inside the column. (3) What are the positions of the feed stream. 4) The minimum number of theoretical plates at total reflux and minimum reflux ratio. * you. can use these: The value of q for cold liquid feed is q=1+HVHLCPL(TBTF) The vake of q for super heated vapor feed is q=0+HVHLCV(TDTF) Vapor-Pressure and Equilibrium Mole Fraction Data for Benzene-Toluene System at 101.325 kPa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


