Question: Please type work, I will thumbs up, thank you. 3. a) Identify the Brnsted-Lowry acid and base for the reaction below. b) Is formic acid
Please type work, I will thumbs up, thank you.
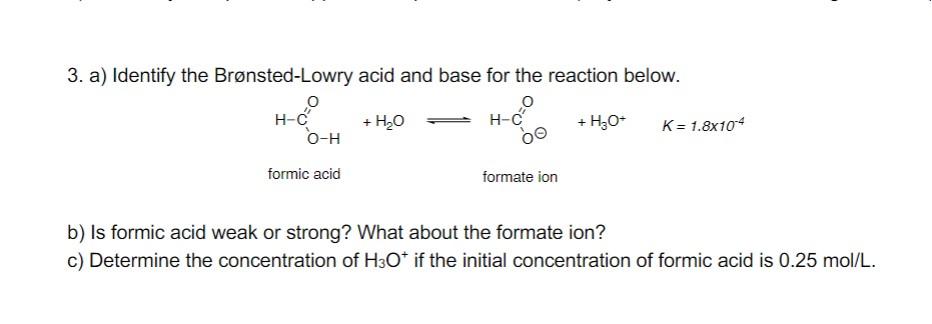
3. a) Identify the Brnsted-Lowry acid and base for the reaction below. b) Is formic acid weak or strong? What about the formate ion? c) Determine the concentration of H3O+if the initial concentration of formic acid is 0.25mol/L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


